D8440
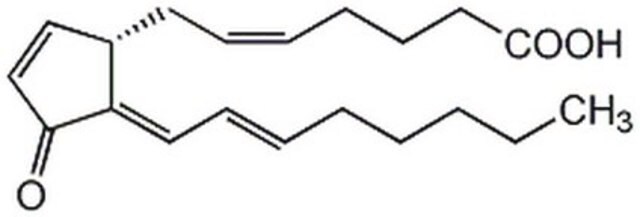
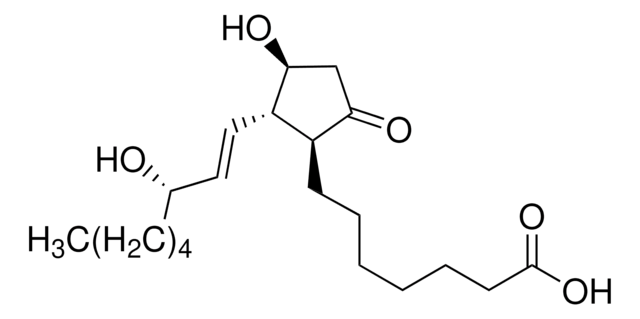
15-Deoxy-Δ12,14-prostaglandin J2
≥95% (HPLC), 1 mg/mL in methyl acetate
동의어(들):
11-Oxoprosta-(5Z,9,12E,14E)-tetraen-1-oic acid, 15-Deoxy-Δ12,14-PGJ2
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H28O3
CAS Number:
Molecular Weight:
316.43
EC Number:
MDL number:
UNSPSC 코드:
12352211
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥95% (HPLC)
양식
liquid
농도
1 mg/mL in methyl acetate
배송 상태
wet ice
저장 온도
−20°C
SMILES string
CCCCC\C=C\C=C1/[C@@H](C\C=C/CCCC(O)=O)C=CC1=O
InChI
1S/C20H28O3/c1-2-3-4-5-6-10-13-18-17(15-16-19(18)21)12-9-7-8-11-14-20(22)23/h6-7,9-10,13,15-17H,2-5,8,11-12,14H2,1H3,(H,22,23)/b9-7-,10-6+,18-13+/t17-/m0/s1
InChI key
VHRUMKCAEVRUBK-GODQJPCRSA-N
일반 설명
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is a prostaglandin J2 (PGJ2) metabolite and a naturally occurring derivative of prostaglandin D2. It is produced during inflammatory processes.
애플리케이션
15-Deoxy-Δ12,14-prostaglandin J2 has been used:
- to study its effect on lipid accumulation, viability/mitochondrial activity, and amount of vasculature in vascularized adipose tissue model
- as a peroxisome proliferator-activated receptor (PPARγ) agonist to activate intestinal fatty acid binding protein (I-FABP)-PPARγ pathway
- as a supplement in culture medium for induced neural stem/progenitor cells (NSPCs) differentiation
생화학적/생리학적 작용
15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) regulates inflammatory response in vivo. 15d-PGJ2 also elicits PPARγ-independent inhibition of nuclear factor (NF)-κB dependent transcription. It acts as an anti-angiogenic factor and triggers endothelial cell apoptosis.
Selective agonist to PPARγ (peroxisome proliferator-activated receptors). Inhibits the proliferation of cancer cell lines that express PPARγ and cyclooxygenase-2 (COX-2).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
15.8 °F - closed cup
Flash Point (°C)
-9 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
J M Launay et al.
Translational psychiatry, 1, e56-e56 (2011-01-01)
Serotonin reuptake inhibitor (SRI) antidepressants such as fluoxetine (Prozac), promote hippocampal neurogenesis. They also increase the levels of the bcl-2 protein, whose overexpression in transgenic mice enhances adult hippocampal neurogenesis. However, the mechanisms underlying SRI-mediated neurogenesis are unclear. Recently, we
Min Zhao et al.
Oncotarget, 7(40), 64690-64701 (2016-09-08)
Accumulating evidence suggests that loss of the renal tubular epithelial phenotype plays an important role in the pathogenesis of renal tubulointerstitial fibrosis. Systemic activation of peroxisome proliferator-activated receptor γ (PPAR-γ) has been shown to be protective against renal fibrosis, although
Mojgan Masoodi et al.
Rapid communications in mass spectrometry : RCM, 20(20), 3023-3029 (2006-09-21)
Prostanoids are potent mediators of many physiological and pathophysiological processes. Of the many analytical methodologies used for their qualitative and quantitative analysis, electrospray tandem mass spectrometry coupled to liquid chromatography (LC/ESI-MS/MS) offers a rapid, sensitive and versatile system applicable to
Gas chromatographic-mass spectrometric measurement of 15-deoxy-delta(12,14)-prostaglandin J(2), the peroxisome proliferator-activated receptor gamma ligand, in urine.
C Thévenon et al.
Clinical chemistry, 47(4), 768-770 (2001-03-29)
Helder Veras Ribeiro Filho et al.
Molecular and cellular endocrinology, 484, 1-14 (2019-02-01)
Nuclear receptors (NRs) are a superfamily of ligand-dependent transcription factors that modulate several biological processes. Traditionally, modulation of NRs has been focused on the development of ligands that recognize and bind to the ligand binding domain (LBD), resulting in activation
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.