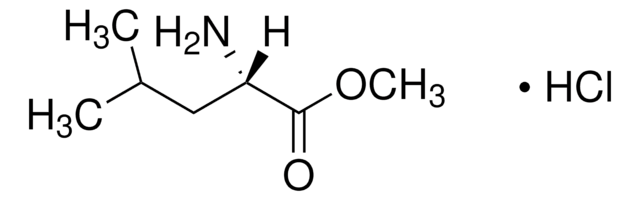
H15403
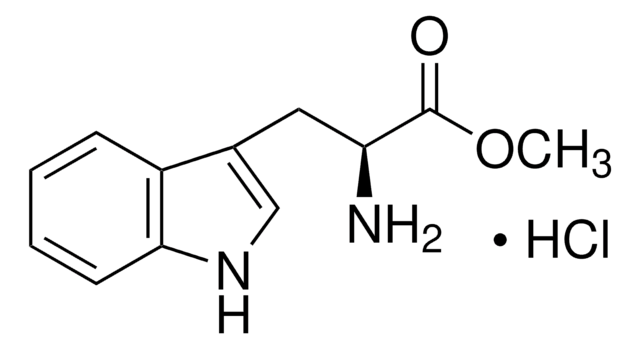
L-Histidine methyl ester dihydrochloride
97%
동의어(들):
(S)-Histidine methyl ester dihydrochloride, Methyl L-histidinate dihydrochloride
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C7H11N3O2 · 2HCl
CAS Number:
Molecular Weight:
242.10
Beilstein:
3572009
EC Number:
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
광학 활성
[α]20/D +9.0°, c = 2 in H2O
반응 적합성
reaction type: solution phase peptide synthesis
색상
white
mp
207 °C (dec.) (lit.)
응용 분야
peptide synthesis
SMILES string
Cl.Cl.COC(=O)[C@@H](N)Cc1c[nH]cn1
InChI
1S/C7H11N3O2.2ClH/c1-12-7(11)6(8)2-5-3-9-4-10-5;;/h3-4,6H,2,8H2,1H3,(H,9,10);2*1H/t6-;;/m0../s1
InChI key
DWAYENIPKPKKMV-ILKKLZGPSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
L-Histidine methyl ester dihydrochloride can be used as a reactant to synthesize:
- Imidazopyridine derivatives by Pictet-Spegler reaction with different aldehydes.
- A metal-chelating ligand, N-methacryloyl-(l)-histidine methyl ester by reacting with methacryloyl chloride.
- Zwitterionic polypeptide derivative by amidation reaction with poly (α,β-L-aspartic acid).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Xiaoyu Su et al.
Chirality, 21(5), 539-546 (2008-08-14)
Two kinds of novel chiral molecular tweezers containing imidazoliums were synthesized from L-alanine, L-phenylalanine, and L-glutamic acid. They are constructed by the chiral imidazolium pincers and two different spacers which are 1,3-bis (bromomethyl)benzene and 2,6-bis(bromomethyl)pyridine, respectively. The enantioselective recognition of
T A Alston et al.
Biochemistry, 26(13), 4082-4085 (1987-06-30)
L-Histidine methyl ester inactivates histidine decarboxylase in a time-dependent manner. The possibility was considered that an irreversible reaction between enzyme and inhibitor occurs [Recsei, P. A., & Snell, E. E. (1970) Biochemistry 9, 1492-1497]. We have confirmed time-dependent inactivation by
V H Vilchiz et al.
Acta crystallographica. Section C, Crystal structure communications, 52 ( Pt 3), 696-698 (1996-03-15)
The title compound, C7H13N3O2(2+).2Cl-, has distances and angles quite similar to those of histidine hydrochloride monohydrate [Donohue & Caron (1964). Acta Cryst. 17, 1178-1180], except for the distances within the ester functionality.
Scott Worley et al.
Proteins, 46(3), 321-329 (2002-02-09)
Histidine decarboxylase (HDC) from Lactobacillus 30a converts histidine to histamine, a process that enables the bacteria to maintain the optimum pH range for cell growth. HDC is regulated by pH; it is active at low pH and inactive at neutral
Y Oya et al.
Mutation research, 198(1), 233-240 (1988-03-01)
An enhancing effect of L-histidine (L-His) was detected on the induction by hydrogen peroxide (H2O2) of chromosomal aberrations of both the chromosome type and the chromatid type, in human embryonic fibroblasts. The maximum efficiency of induction was about 8-fold higher
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.