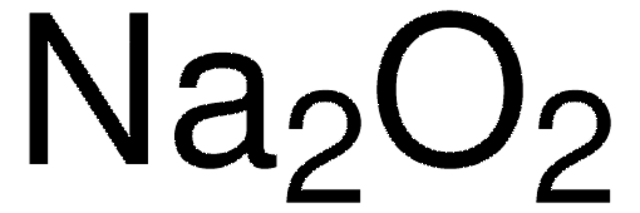추천 제품
Grade
reagent grade
Quality Level
분석
97%
양식
granular
반응 적합성
reagent type: oxidant
입자 크기
+140 mesh
pH
12.8 (20 °C, 100 g/L)
mp
460 °C (dec.) (lit.)
SMILES string
[Na+].[Na+].[O-][O-]
InChI
1S/2Na.O2/c;;1-2/q2*+1;-2
InChI key
PFUVRDFDKPNGAV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sodium peroxide (Sodium superoxide) has been synthesized by the matrix reaction of sodium atoms and oxygen molecules at high dilution in argon. Its IR spectral studies have been reported. It is a strong oxidizing agent.
Sodium peroxide is a strong oxidizing and bleaching agent mainly used in bleaching papers and textiles. It is an effective oxidizing flux for the decomposition of minerals and ores.
애플리케이션
Sodium peroxide may be used as an oxidant for:
conversion of Δ4-3-ketosteroids to Δ4-3,6-diones
oxidation of alduronic and glyculosonic acids
conversion of Δ4-3-ketosteroids to Δ4-3,6-diones
oxidation of alduronic and glyculosonic acids
Sodium peroxide may be used for the isotope dilution inductively coupled plasma mass spectrometric quantification of Ru, Pd, Ir and Pt in various geological samples. It may be employed for the inductively coupled plasma mass spectrometric quantification of rare earth elements (such as Y, Th, Zr, Hf, Nb and Ta) in geological reference materials.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Ox. Sol. 1 - Skin Corr. 1A
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Oxidation with sodium peroxide Direct introduction of a ? carbonyl group into a, ? unsaturated ketosteroids
Tetrahedron Letters, 22(51), 5127-5128 (1981)
Oxidation of sodium salts of alduronic and glyculosonic acids by sodium peroxide.
Isbell HS, et al.
Carbohydrate Research, 36(2), 283-291 (1974)
Wiberg E and Wiberg N
Inorganic Chemistry, 502-502 (2001)
Eagleson M.
Concise Encyclopedia Chemistry, 1080-1080 (1994)
Determination of platinum, palladium, ruthenium and iridium in geological samples by isotope dilution inductively coupled plasma mass spectrometry using a sodium peroxide fusion and tellurium coprecipitation
Enzweiler J, et al.
Analyst, 120(5), 1391-1396 (1995)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.