86853
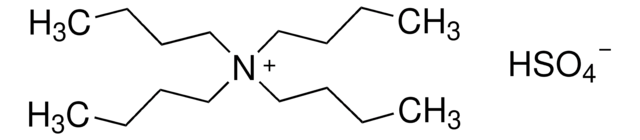
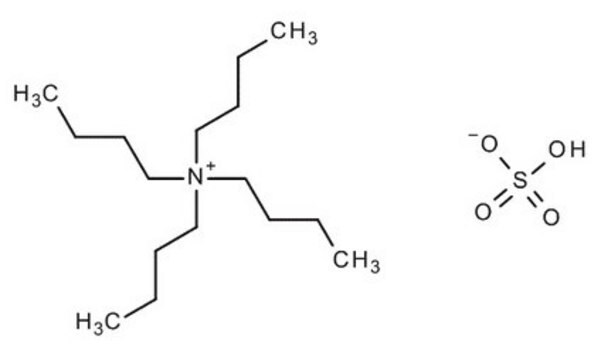
Tetrabutylammonium bisulfate
suitable for ion pair chromatography, LiChropur™, ≥99.0%
동의어(들):
Tetrabutylammonium hydrogen sulfate
About This Item
추천 제품
Grade
reagent grade
Quality Level
설명
cationic
분석
≥99.0% (T)
≥99.0%
양식
crystals
품질
LiChropur™
기술
ion pair chromatography: suitable
mp
171-174 °C
음이온 미량물
bromide (Br-): ≤50 mg/kg
λ
10 % in H2O
UV 흡수
λ: 210 nm Amax: 0.06
λ: 220 nm Amax: 0.05
λ: 230 nm Amax: 0.03
λ: 260 nm Amax: 0.02
λ: 500 nm Amax: 0.02
적합성
corresponds to standard for RP gradient test
corresponds to standard for filter test
SMILES string
OS([O-])(=O)=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.H2O4S/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-5(2,3)4/h5-16H2,1-4H3;(H2,1,2,3,4)/q+1;/p-1
InChI key
SHFJWMWCIHQNCP-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point (°F)
348.1 °F - closed cup
Flash Point (°C)
175.6 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
프로토콜
Because reversed-phase HPLC is primarily dependent on hydrophobic interactions between the stationary phase and analyte, ion pairing is occasionally necessary to obtain sufficient retention of polar, ionizable compounds.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.