86833
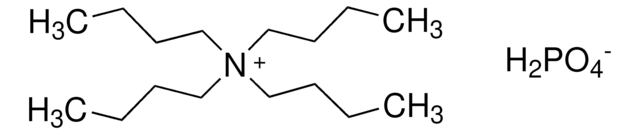
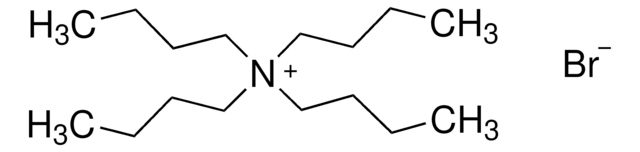
Tetrabutylammonium phosphate monobasic
puriss., 99% (T)
동의어(들):
Tetrabutylammonium dihydrogen phosphate
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4N[OP(OH)2O]
CAS Number:
Molecular Weight:
339.45
Beilstein:
5196532
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.21
추천 제품
grade
puriss.
Quality Level
분석
99% (T)
양식
powder
mp
151-154 °C (lit.)
SMILES string
OP(O)([O-])=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.H3O4P/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-5(2,3)4/h5-16H2,1-4H3;(H3,1,2,3,4)/q+1;/p-1
InChI key
ARRNBPCNZJXHRJ-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Tetrabutylammonium phosphate monobasic is a synthetic reagent. It can serve as a pH buffer in various chemical and biological applications.
애플리케이션
Tetrabutylammonium phosphate monobasic is the suitable reagent used in the following studies:
- Resolution of disaccharides reverse-phase ion-pairing HPLC (RPIP-HPLC).
- Disaccharide analysis of 35S- heparan sulfate (HS).
- As folding buffer for the dissolution of desalted oligonucleotides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Vaibhav Tiwari et al.
Journal of virology, 80(18), 8970-8980 (2006-08-31)
Herpes simplex virus type 1 (HSV-1) infection of the corneal stroma remains a major cause of blindness. Primary cultures of corneal fibroblasts (CF) were tested and found susceptible to HSV-1 entry, which was confirmed by deconvolution imaging of infected cells.
Robert D Gray et al.
Nucleic acids research, 36(12), 4191-4203 (2008-06-24)
Cation-induced folding into quadruplex structures for three model human telomeric oligonucleotides, d[AGGG(TTAGGG)(3)], d[TTGGG(TTAGGG)(3)A] and d[TTGGG(TTAGGG)(3)], was characterized by equilibrium titrations with KCl and NaCl and by multiwavelength stopped flow kinetics. Cation binding was cooperative with Hill coefficients of 1.5-2.2 in
Polymerisable squaramide receptors for anion binding and sensing.
Manesiotis P, et al.
Journal of Material Chemistry C, 2(42), 8990-8995 (2014)
J Liu et al.
The Journal of biological chemistry, 274(53), 38155-38162 (1999-12-23)
3-O-Sulfation of glucosamine by heparan sulfate D-glucosaminyl 3-O-sulfotransferase (3-OST-1) is the key modification in anticoagulant heparan sulfate synthesis. However, the heparan sulfates modified by 3-OST-2 and 3-OST-3A, isoforms of 3-OST-1, do not have anticoagulant activity, although these isoforms transfer sulfate
Guoqing Xia et al.
The Journal of biological chemistry, 277(40), 37912-37919 (2002-07-26)
Heparan sulfate 3-O-sulfotransferase transfers sulfate to the 3-OH position of a glucosamine residue of heparan sulfate (HS) to form 3-O-sulfated HS. The 3-O-sulfated glucosamine residue contributes to two important biological functions of HS: binding to antithrombin and thereby carrying anticoagulant
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.