730998
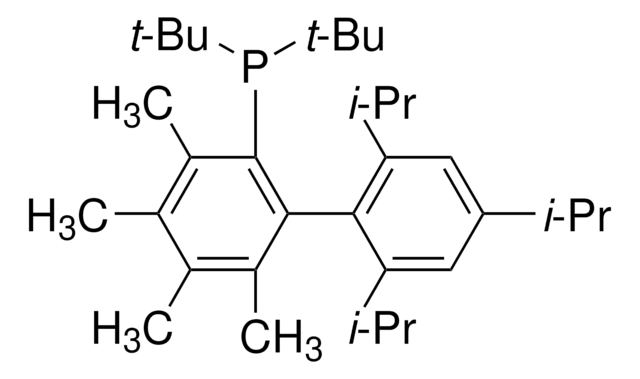
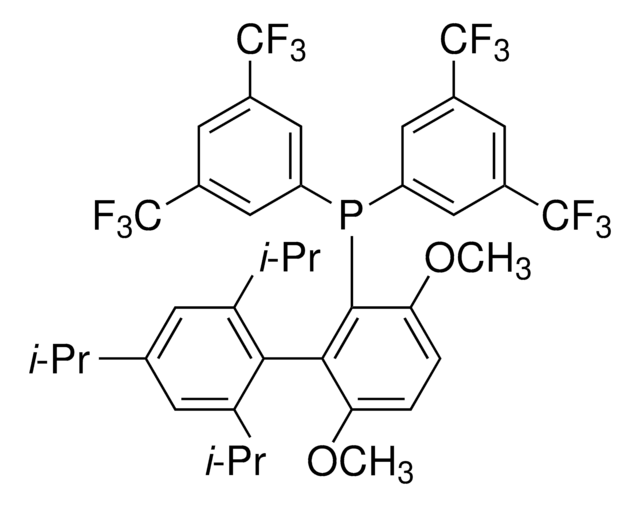
tBuBrettPhos
97%
동의어(들):
t-Bu Brett Phos, t-BuBrett-Phos, tertButylBrettPhos, 2-(Di-tert-butylphosphino)-2′,4′,6′- triisopropyl-3,6-dimethoxy-1,1′-biphenyl, t-BuBrett Phos, t-BuBrettPhos, [3,6-Dimethoxy-2′,4′,6′-tris(1-methylethyl) [1,1′-biphenyl]-2-yl]bis(1,1-dimethylethyl)phosphine, tert-ButylBrettPhos
About This Item
추천 제품
Quality Level
분석
97%
양식
solid
반응 적합성
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Fluorinations
mp
166-170 °C
작용기
phosphine
SMILES string
COc1ccc(OC)c(c1P(C(C)(C)C)C(C)(C)C)-c2c(cc(cc2C(C)C)C(C)C)C(C)C
InChI
1S/C31H49O2P/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12/h15-21H,1-14H3
InChI key
REWLCYPYZCHYSS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
tBuBrettPhos is a phosphine ligand widely used in palladium-catalyzed cross-coupling reactions.
애플리케이션
- Buchwald-Hartwig amination and C-O coupling
- Suzuki, Negishi, Stille, Hiyama, Sonogashira cross-couplings
- α-Arylation reaction
New Applications:
- Conversion of aryl and vinyl triflates to bromides and chlorides
- Conversion of aryl triflates to aryl fluorides
- O-Arylation of ethyl acetohydroximate
- Conversion of aryl chlorides and sulfonates to nitroaromatics
특징 및 장점
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
법적 정보
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.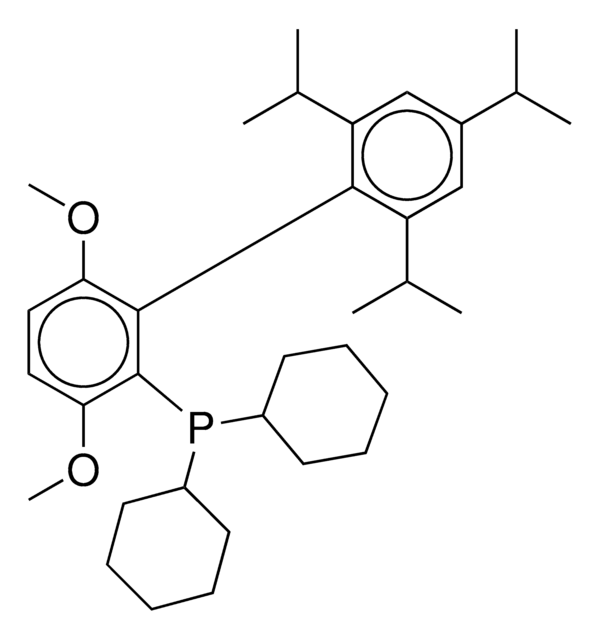


![5-(Di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole 97%](/deepweb/assets/sigmaaldrich/product/structures/137/599/8b2f4b58-3384-40aa-9295-0887f7985525/640/8b2f4b58-3384-40aa-9295-0887f7985525.png)