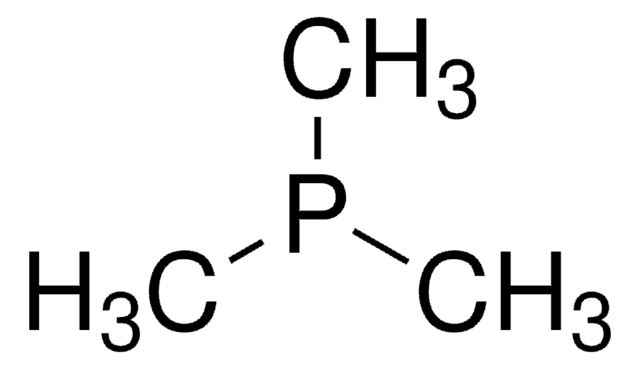
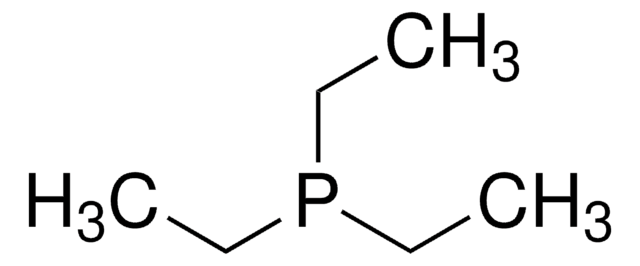
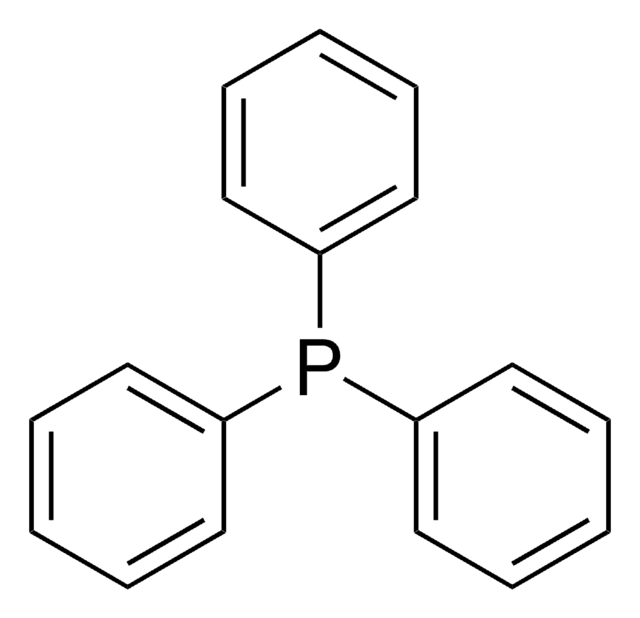
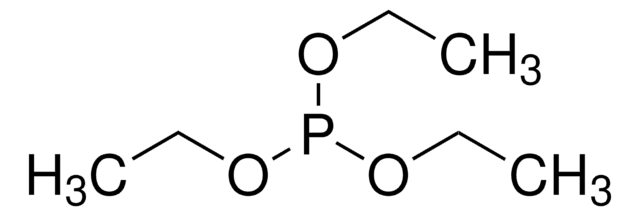
추천 제품
Quality Level
분석
99%
양식
liquid
반응 적합성
reagent type: ligand
reaction type: Arylations
refractive index
n20/D 1.456 (lit.)
bp
127-128 °C (lit.)
density
0.802 g/mL at 20 °C (lit.)
작용기
phosphine
SMILES string
CCP(CC)CC
InChI
1S/C6H15P/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
RXJKFRMDXUJTEX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Triethylphosphine is generally used as a ligand in the organometallic chemistry. It can be used in:
- Synthesis of the tetrahedrally coordinated L3Fe-Nx complex with a terminal nitride group.
- Synthesis of (PEt3)2Ni(η2-C14H10), a source of the reactive Ni(PEt3)2 moiety.
- Synthesis of dinuclear rhodium complexes with triethyllphosphane bridges.
기타 정보
This container should be opened only by a technically qualified person.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1B
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
A Tetrahedrally Coordinated L3Fe? N x Platform that Accommodates Terminal Nitride (FeIV? N) and Dinitrogen (FeI? N2? FeI) Ligands.
Betley T A and Peters J C
Journal of the American Chemical Society, 126(20), 6252-6254 (2004)
Unexpected Intermediates and Products in the C? F Bond Activation of Tetrafluorobenzenes with a Bis (triethylphosphine) Nickel Synthon: Direct Evidence of a Rapid and Reversible C? H Bond Activation by Ni (0).
Johnson S A, et al.
Journal of the American Chemical Society, 130(51), 17278-17280 (2008)
Breaking the Rule: Synthesis and Molecular Structure of Dinuclear Rhodium Complexes with Bridging and Semibridging Trialkylphosphane Ligands.
Pechmann T, et al.
Angewandte Chemie (International Edition in English), 39(21), 3909-3911 (2000)
Quintin R Sheridan et al.
The journal of physical chemistry. B, 120(46), 11951-11960 (2016-10-27)
A combination of X-ray scattering experiments and molecular dynamics simulations were conducted to investigate the structure of ionic liquids (ILs) which chemically bind CO
Sarah A Weicker et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(37), 13027-13034 (2015-08-01)
Silyl triflates of the form R4-n Si(OTf)n (n=1, 2; OTf=OSO3 CF3 ) are shown to activate carbon dioxide when paired with bulky alkyl-substituted Group 15 bases. Combinations of silyl triflates and 2,2,6,6-tetramethylpiperidine react with CO2 to afford silyl carbamates via a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.