940194
Lithium peroxide

99.9% trace metals basis, battery grade
Synonyme(s) :
Dilithium peroxide, Lithium(I) peroxide
About This Item
Produits recommandés
Qualité
battery grade
Niveau de qualité
Essai
99.9% trace metals basis
Forme
powder
Solubilité
acetic acid: soluble
alcohol: insoluble
water: soluble
Densité
2.32 g/cm3 (lit.)
Application(s)
battery manufacturing
Chaîne SMILES
[Li+].[Li+].[O-][O-]
InChI
1S/2Li.O2/c;;1-2/q2*+1;-2
Clé InChI
HPGPEWYJWRWDTP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Lithium peroxide is an inorganic compound with the composition Li2O2 that takes the form of a white powder. Chemically, lithium peroxide is strongly basic and a powerful oxidizing agent. At room temperature, it is stable in air, showing neither hygroscopic properties nor a tendency to absorb carbon dioxide. At elevated temperatures in air, Li2O2 and CO2 establish a reversible chemical equilibrium with lithium carbonate and oxygen. At even higher temperatures, ~340-450 °C, lithium peroxide decomposes to lithium oxide (Li2O) and oxygen gas.
Application
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A
Code de la classe de stockage
5.1B - Oxidizing hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
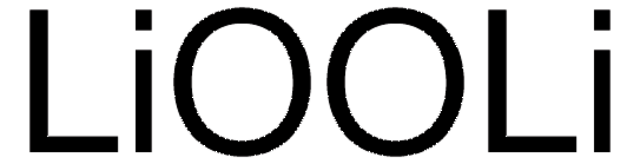



![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)


