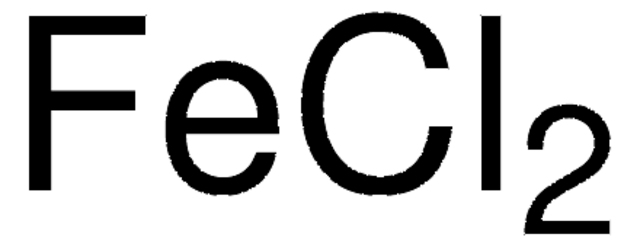939935
Iron(II) chloride anhydrous

≥99.99% trace metals basis
Synonym(s):
Iron(II) chloride, Ferrous chloride, Iron dichloride
About This Item
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
powder or crystals
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤100 ppm (trace metals analysis)
mp
677 °C (lit.)
solubility
water: soluble
density
3.16 g/mL at 25 °C (lit.)
3.16 g/mL at 25 °C
anion traces
chlorate, nitrate (as NO3-): ≤20 ppm
sulfate (SO42-): ≤20 ppm
≤50 ppm (oxygen)
cation traces
Al: ≤10 ppm
B: ≤10 ppm
Ba: ≤10 ppm
Ca: ≤10 ppm
Co: ≤10 ppm
Cr: ≤10 ppm
Cu: ≤10 ppm
K: ≤10 ppm
Mg: ≤10 ppm
Mn: ≤10 ppm
Na: ≤10 ppm
Ni: ≤10 ppm
Si: ≤10 ppm
Ti: ≤10 ppm
Zn: ≤10 ppm
greener alternative category
storage temp.
2-30°C
SMILES string
Cl[Fe]Cl
InChI
1S/2ClH.Fe/h2*1H;/q;;+2/p-2
InChI key
NMCUIPGRVMDVDB-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
- Anhydrous
- Low water content
- Low Oxygen content
-Perfect use for molten salt reactors
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service