1559006
USP
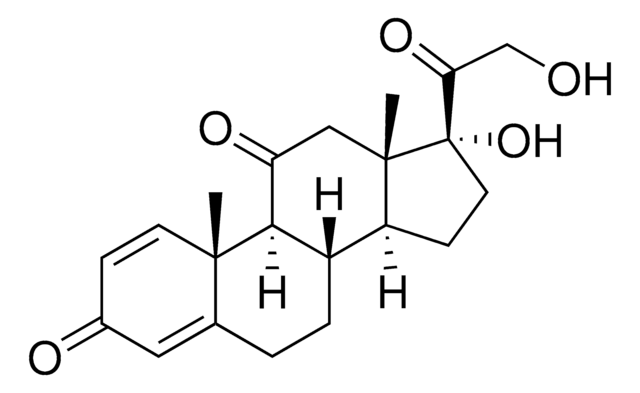
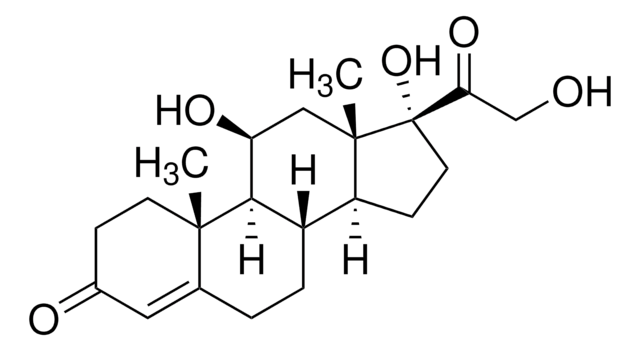
Prednisone
United States Pharmacopeia (USP) Reference Standard
동의어(들):
1,4-Pregnadiene-17α,21-diol-3,11,20-trione, 1-Cortisone, 17α,21-Dihydroxy-1,4-pregnadiene-3,11,20-trione, Dehydrocortisone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H26O5
CAS Number:
Molecular Weight:
358.43
Beilstein:
2065301
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
prednisone
제조업체/상표
USP
mp
236-238 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
O=C1C=C[C@@]2(C)C(CC[C@]([C@@](CC[C@@]3(C(CO)=O)O)([H])[C@]3(C)C4)([H])[C@]2([H])C4=O)=C1
InChI
1S/C21H26O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-15,18,22,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1
InChI key
XOFYZVNMUHMLCC-ZPOLXVRWSA-N
유전자 정보
human ... NR3C1(2908)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Prednisone USP reference standard for specified quality tests and assay use.
Also used to prepare standard, standard stock and internal standard solutions for the assay and performance tests according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard, standard stock and internal standard solutions for the assay and performance tests according to the given below monographs of United States Pharmacopeia (USP):
- Prednisone
- Prednisone Tablets
- Prednisone Oral Solution
- Hydrocortisone Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Prednisone Oral Solution
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 30(1), 3671-3671 (2020)
Florentien D O de Steenwinkel et al.
Arthritis & rheumatology (Hoboken, N.J.), 66(7), 1705-1711 (2014-03-01)
Active rheumatoid arthritis (RA) during pregnancy and the presence of rheumatoid factor (RF) or anti-citrullinated protein antibodies (ACPAs) are associated with lower birth weight of the child. Moreover, treatment of the mothers with prednisone may shorten the gestational age at
O Teuffel et al.
Leukemia, 25(8), 1232-1238 (2011-04-30)
This systematic review and meta-analysis compared the efficacy and toxicity of dexamethasone (DEX) versus prednisone (PRED) for induction therapy in childhood acute lymphoblastic leukemia (ALL). We searched biomedical literature databases and conference proceedings for randomized controlled trials comparing DEX and
Ronald de Wit
European journal of cancer (Oxford, England : 1990), 41(4), 502-507 (2005-03-02)
Until now, the use of systemic chemotherapy for advanced androgen-independent prostate cancer has had very little to offer to patients. However, in 2004, two large randomised trials investigating docetaxel vs. mitoxantrone have both demonstrated survival improvements, and, in one of
Karim Fizazi et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33(7), 723-731 (2015-01-28)
Orteronel (TAK-700) is an investigational, nonsteroidal, reversible, selective 17,20-lyase inhibitor. This study examined orteronel in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel therapy. In our study, 1,099 men were randomly assigned in a 2:1 schedule to receive
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.