P2900000
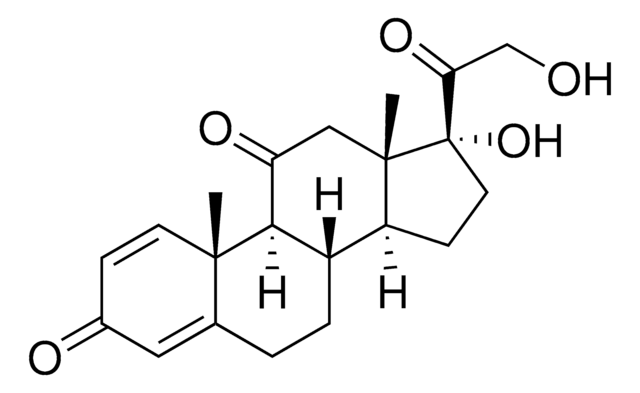
Prednisone
European Pharmacopoeia (EP) Reference Standard
동의어(들):
1,4-Pregnadiene-17α,21-diol-3,11,20-trione, 1-Cortisone, 17α,21-Dihydroxy-1,4-pregnadiene-3,11,20-trione, Dehydrocortisone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H26O5
CAS Number:
Molecular Weight:
358.43
Beilstein:
2065301
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
prednisone
제조업체/상표
EDQM
mp
236-238 °C (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
O=C1C=C[C@@]2(C)C(CC[C@]([C@@](CC[C@@]3(C(CO)=O)O)([H])[C@]3(C)C4)([H])[C@]2([H])C4=O)=C1
InChI
1S/C21H26O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h5,7,9,14-15,18,22,26H,3-4,6,8,10-11H2,1-2H3/t14-,15-,18+,19-,20-,21-/m0/s1
InChI key
XOFYZVNMUHMLCC-ZPOLXVRWSA-N
유전자 정보
human ... NR3C1(2908)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Prednisone EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Karim Fizazi et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33(7), 723-731 (2015-01-28)
Orteronel (TAK-700) is an investigational, nonsteroidal, reversible, selective 17,20-lyase inhibitor. This study examined orteronel in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel therapy. In our study, 1,099 men were randomly assigned in a 2:1 schedule to receive
Fred Saad et al.
The Lancet. Oncology, 16(3), 338-348 (2015-02-24)
Orteronel is an investigational, partially selective inhibitor of CYP 17,20-lyase in the androgen signalling pathway, a validated therapeutic target for metastatic castration-resistant prostate cancer. We assessed orteronel in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. In this phase 3, double-blind
Lindsay Sonstein et al.
The American journal of medicine, 127(11), 1097-1104 (2014-06-15)
Clinical practice guidelines recommend 40-60 mg of prednisone equivalent for 10-14 days for patients with acute exacerbations of chronic obstructive pulmonary disease (COPD). However, the amount of corticosteroid prescribed varies widely in clinical practice. Using the electronic health record, we
Claudine Angela Blum et al.
Lancet (London, England), 385(9977), 1511-1518 (2015-01-23)
Clinical trials yielded conflicting data about the benefit of adding systemic corticosteroids for treatment of community-acquired pneumonia. We assessed whether short-term corticosteroid treatment reduces time to clinical stability in patients admitted to hospital for community-acquired pneumonia. In this double-blind, multicentre
Katalin A Wilkinson et al.
Journal of immunology (Baltimore, Md. : 1950), 194(4), 1748-1754 (2015-01-16)
Tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) frequently complicates combined antiretroviral therapy and antituberculosis therapy in HIV-1-coinfected tuberculosis patients. The immunopathological mechanisms underlying TB-IRIS are incompletely defined, and improved understanding is required to derive new treatments and to reduce associated morbidity
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.