추천 제품
solubility
alcohol: soluble
Quality Level
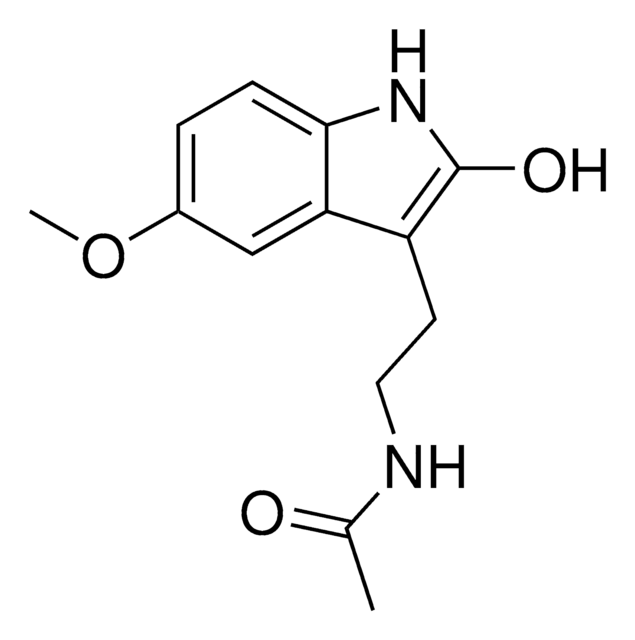
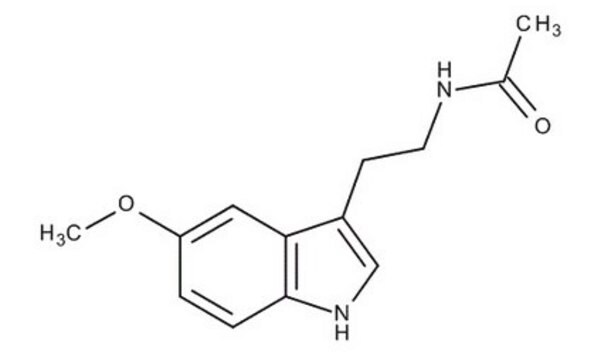
SMILES string
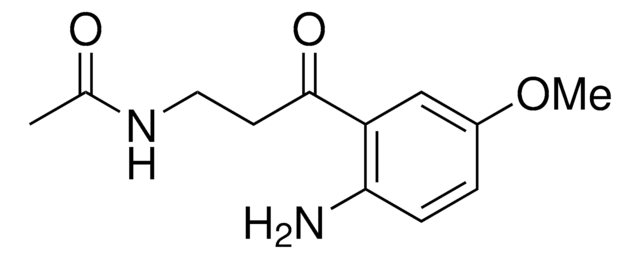
COc1cc2c(CCNC(C)=O)c[nH]c2cc1O
InChI
1S/C13H16N2O3/c1-8(16)14-4-3-9-7-15-11-6-12(17)13(18-2)5-10(9)11/h5-7,15,17H,3-4H2,1-2H3,(H,14,16)
InChI key
OMYMRCXOJJZYKE-UHFFFAOYSA-N
유전자 정보
human ... MTNR1A(4543) , MTNR1B(4544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
6-Hydroxymelatonin (6-OHM) is a melatonin metabolite. It is produced in the liver by the action of cytochrome P450 enzyme as well as by photodegradation of melatonin. In the central nervous system, it exists as a sulfated form. 6-OHM is a partial melatonin receptor MT2 agonist.
애플리케이션
6-Hydroxymelatonin has been used as a melatonin derivative to test its protective effects in ultra violet B (UVB)-induced oxidative stress melanocytes and keratinocytes.
생화학적/생리학적 작용
6-Hydroxymelatonin (6-OHM) is an antioxidant with a free radical scavenging role. Like melatonin, it reduces the impact of UVB-induced oxidative stress in melanocytes. It also aids protection during iron (Fe2+)-induced neurotoxicity. 6-OHM effectively reduces lipid peroxidation and superoxide anion production induced by potassium cyanide (KCN).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Deepa S Maharaj et al.
Journal of neurochemistry, 96(1), 78-81 (2005-11-23)
Oxidative damage of biological macromolecules is a hallmark of most neurodegenerative disorders such as Alzheimer, Parkinson and diffuse Lewy body diseases. Another important phenomenon involved in these disorders is the alteration of iron homeostasis, with an increase in iron levels.
Xuwan Liu et al.
American journal of physiology. Heart and circulatory physiology, 283(1), H254-H263 (2002-06-14)
The present study was designed to explore the protective effects of melatonin and its analogs, 6-hydroxymelatonin and 8-methoxy-2-propionamidotetralin, on the survival of doxorubicin-treated mice and on doxorubicin-induced cardiac dysfunction, ultrastructural alterations, and apoptosis in mouse hearts. Whereas 60% of the
Katsuhisa Sakano et al.
Biochemical pharmacology, 68(9), 1869-1878 (2004-09-29)
Melatonin, an indolic pineal hormone, is produced primarily at night in mammals and is important in controlling biological rhythms. Although melatonin is known to be effective as a free radical scavenger and has an anti-cancer effect, carcinogenic properties have also
G Facciolá et al.
European journal of clinical pharmacology, 56(12), 881-888 (2001-04-25)
The present study was carried out to identify the cytochrome P450 enzyme(s) involved in the 6-hydroxylation and O-demethylation of melatonin. The formation kinetics of 6-hydroxymelatonin and N-acetylserotonin were determined using human liver microsomes and cDNA yeast-expressed human enzymes (CYP1A2, 2C9
S Härtter et al.
Therapeutic drug monitoring, 23(3), 282-286 (2001-05-22)
Melatonin has recently garnered interest as a possible treatment for sleep disorders, and this has created a desire for appropriate pharmacokinetic studies. No method has yet been published that can measure the concentrations of both melatonin and its main metabolite
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.