286583
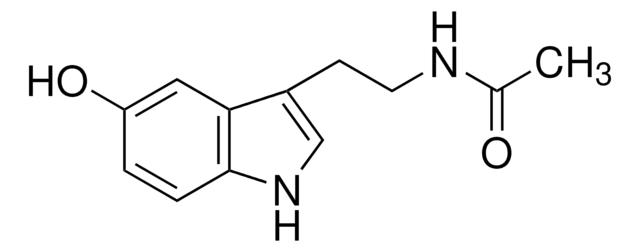
5-Methoxytryptamine
97%
동의어(들):
2-(5-Methoxyindol-3-yl)ethylamine, 3-(2-Aminoethyl)-5-methoxyindole, 5-MOT, 5-Methoxyindole-3-ethanamine, Deacetylmelatonin, NSC 56422
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C11H14N2O
CAS Number:
Molecular Weight:
190.24
Beilstein:
145587
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
solid
mp
121-123 °C (lit.)
작용기
amine
SMILES string
COc1ccc2[nH]cc(CCN)c2c1
InChI
1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3
InChI key
JTEJPPKMYBDEMY-UHFFFAOYSA-N
유전자 정보
human ... HTR1A(3350) , HTR2A(3356) , HTR2C(3358)
rat ... Htr2a(29595) , Htr2c(25187) , Htr7(65032)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied.
애플리케이션
5-Methoxytryptamine was used as an agonist in the study of pharmacological profile of the 5-hydroxytryptamine 1 receptor.
Reactant for preparation of:
- Carboline disaccharide domain of shishijimicin A
- Melatonin analogs for the reduction of intraocular pressure
- 5-HT4 receptor ligands
- inhibitors of sortase A and isocitrate lyase
- Therapeutic agents for treatment of ischemia/reperfusion (I/R) injury
- Aurora and epidermal growth factor receptor kinase inhibitors
- Agents for the treatment of human papillomavirus infection
- Manzamine analogues for the control of neuroinflammation and cerebral infections
- Inhibitors of pro-inflammatory cytokines
- Tacrine-melatonin hybrids as multifunctional agents for alzheimer′s disease
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Amir Hanna-Elias et al.
European journal of medicinal chemistry, 44(7), 2952-2959 (2009-02-19)
Twenty-three indole-3-methanamines were designed, synthesized and evaluated as ligands for the 5-HT(4) receptor. Compounds I-d, I-j, I-o, I-q and I-u showed good affinity at 100 microM and I-o was found to be only 5-fold less potent than the agonists serotonin
Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes.
Janjetovic Z, Nahmias ZP, Hanna S, et al.
Journal of Pineal Research, 57(1), 90-102 (2014)
Rut Vleugels et al.
PloS one, 8(5), e65052-e65052 (2013-06-07)
Serotonin (5-hydroxytryptamine, 5-HT) is known for its key role in modulating diverse physiological processes and behaviors by binding various 5-HT receptors. However, a lack of pharmacological knowledge impedes studies on invertebrate 5-HT receptors. Moreover, pharmacological information is urgently needed in
Brian J Prendergast
Endocrinology, 151(2), 714-721 (2009-12-08)
Environmental day length drives nocturnal pineal melatonin secretion, which in turn generates or entrains seasonal cycles of physiology, reproduction, and behavior. In mammals, melatonin (MEL) binds to a number of receptor subtypes including high-affinity (MT1 and MT2) and low-affinity (MT3
R Hardeland
Reproduction, nutrition, development, 39(3), 399-408 (1999-07-27)
Melatonin seems to be an almost ubiquitous substance, which has been detected not only in metazoans, but also in all major non-metazoan taxa investigated, including bacteria, dinoflagellates, euglenoids, trypanosomids, fungi, rhodophyceans, pheophyceans, chlorophyceans and angiosperms. Despite its vast abundance, little
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.