모든 사진(3)
About This Item
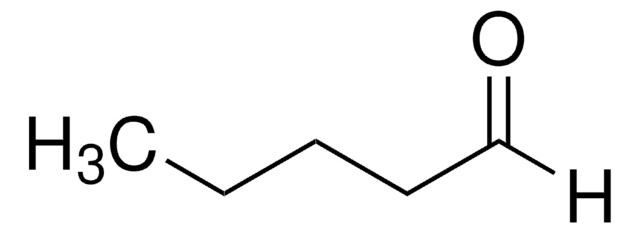
Linear Formula:
CH3CH2CH2CHO
CAS Number:
Molecular Weight:
72.11
Beilstein:
506061
EC Number:
MDL number:
UNSPSC 코드:
12352114
PubChem Substance ID:
NACRES:
NA.21
bp:
75 °C (lit.)
vapor pressure:
90 mmHg ( 20 °C)
추천 제품
vapor density
2.5 (vs air)
Quality Level
vapor pressure
90 mmHg ( 20 °C)
분석
≥99.0%
autoignition temp.
390 °F
expl. lim.
12.5 %
불순물
≤0.30% (water)
refractive index
n20/D 1.380 (lit.)
bp
75 °C (lit.)
mp
−96 °C (lit.)
solubility
water: soluble 50 g/L at 20 °C
density
0.8 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)CCC
InChI
1S/C4H8O/c1-2-3-4-5/h4H,2-3H2,1H3
InChI key
ZTQSAGDEMFDKMZ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Butyraldehyde (n-Butyraldehyde), an aliphatic aldehyde, can be synthesized by dehydrogenation of butanol in the presence of zinc catalyst. It is an important precursor for preparing polyvinylbutyral. The mechanism of pyrolysis of butyraldehyde in the gas-phase has been described. The kinetic study of the condensation reaction between butyraldehyde and poly(vinyl alcohol) indicates retardation effect.
애플리케이션
Butyraldehyde (n-Butyraldehyde) may be used in the preparation of 2-ethylhexanal (2EH) via hydrogenation in the presence of tetraamine palladium(II) chloride supported on the potassium ion-exchanged zeolite X (Pd/KXW) and on the potassium ion-added zeolite X (Pd/KXU).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
<50.0 °F - Pensky-Martens closed cup
Flash Point (°C)
< 10 °C - Pensky-Martens closed cup
이미 열람한 고객
Highly selective Pd/KX catalysts for low-pressure one-step synthesis of 2-ethylhexanal from n-butyraldehyde and hydrogen.
Ko AN, et al.
Catalysis Letters, 54(4), 207-210 (1998)
Retardation effect in acetalization of poly (vinyl alcohol) with butyraldehyde.
Rumyantsev M, et al.
Eur. Polymer J., 49(6), 1698-1706 (2013)
Thermal decomposition products of butyraldehyde.
Hatten CD, et al.
J. Chem. Phys. , 139(21), 214303-214303 (2013)
Kinetic studies on dehydrogenation of butanol to butyraldehyde using zinc oxide as catalyst.
Raizada VK, et al.
Journal of Chemical Technology and Biotechnology, 56(3), 265-270 (1993)
N Gregory et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 44(8), 1223-1227 (2006-03-17)
Bovine soles and shavings from the heel were used in laboratory tests that examined the softening and swelling effects of rainwater, cow slurry (faeces plus urine), urine, silage effluent, and washings from recently laid concrete. Formalin, glutaraldehyde and butyraldehyde were
프로토콜
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.