모든 사진(2)
About This Item
Linear Formula:
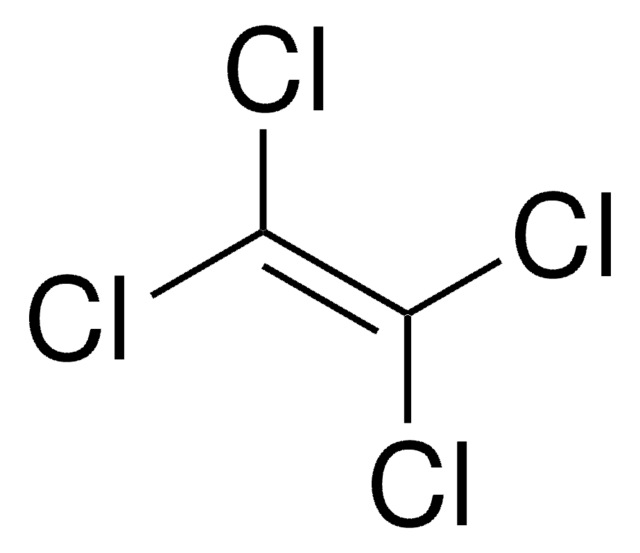
CCl2=CCl2
CAS Number:
Molecular Weight:
165.83
Beilstein:
1361721
EC Number:
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.04
bp:
121 °C (lit.)
vapor pressure:
13 mmHg ( 20 °C)
19 mmHg ( 25 °C)
19 mmHg ( 25 °C)
추천 제품
Grade
ACS reagent
Quality Level
vapor density
5.83 (vs air)
vapor pressure
13 mmHg ( 20 °C)
19 mmHg ( 25 °C)
분석
≥99.0%
양식
liquid
기술
FTIR: suitable
불순물
≤0.05% water
증발 잔류물
≤0.0005%
색상
APHA: ≤10
refractive index
n20/D 1.505 (lit.)
bp
121 °C (lit.)
mp
−22 °C (lit.)
solubility
water: soluble 0.15 g/L at 25 °C
density
1.623 g/mL at 25 °C (lit.)
SMILES string
Cl\C(Cl)=C(\Cl)Cl
InChI
1S/C2Cl4/c3-1(4)2(5)6
InChI key
CYTYCFOTNPOANT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Tetrachloroethylene (perchloroethylene, PCE), is a chlorinated ethylene compound commonly used as a dry cleaning and degreasing solvent. It shows IR transparency as it has no C–H bonds making it an ideal solvent for IR spectroscopy. PCE is a man-made pollutant which is difficult to degrade. It is a ground water contaminant which has adverse effect on human health due to its potential toxicity and carcinogenicity. Some of the methods proposed for its degradation are Fenton oxidation treatment, reductive dehalogenation under methanogenic condition, and reduction using zero valent metal ions. One of the methods reported for its synthesis is by reacting ethylene dichloride and chlorine.
애플리케이션
Tetrachloroethylene may be used as a film-forming electrolyte additive in the manufacture of lithium ion batteries. It may also be used as an extractant for the estimation of oil and grease in water by Fourier transform infrared spectroscopy (FT-IR).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
No data available
Flash Point (°C)
No data available
이미 열람한 고객
Validation of a FT-IR method for the determination of oils and grease in water using tetrachloroethylene as the extraction solvent.
Farmaki E, et al.
Desalination, 210(1), 52-60 (2007)
Tetrachloroethylene as new film-forming additive to propylene carbonate-based electrolytes for lithium ion batteries with graphitic anode.
Hu Y, et al.
Solid State Ionics, 176(1), 53-56 (2005)
Decomposition of aqueous tetrachloroethylene by Fenton oxidation treatment.
Yoshida M, et al.
Water Science and Technology, 42(1-2), 203-208 (2000)
Robert DM and Murphy B.
Chlorinated Solvents: A Forensic Evaluation, 84-85 (2013)
D Ryoo et al.
Nature biotechnology, 18(7), 775-778 (2000-07-11)
Tetrachloroethylene (PCE) is thought to have no natural source, so it is one of the most difficult contaminants to degrade biologically. This common groundwater pollutant was thought completely nonbiodegradable in the presence of oxygen. Here we report that the wastewater
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.