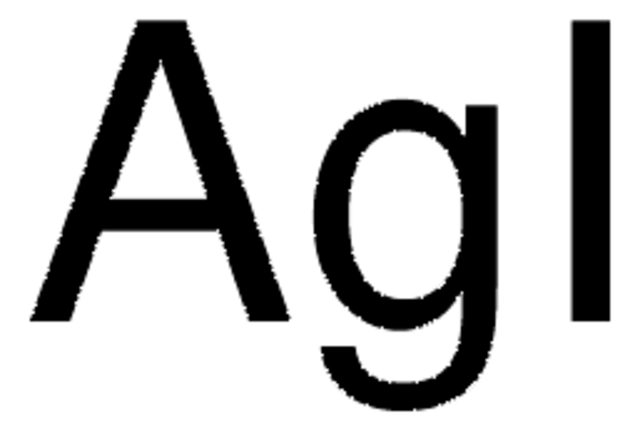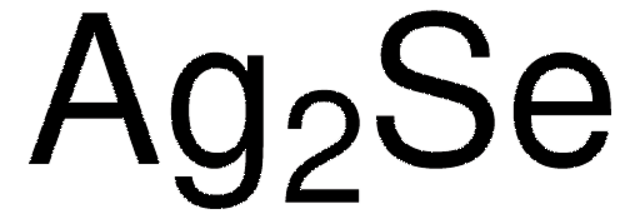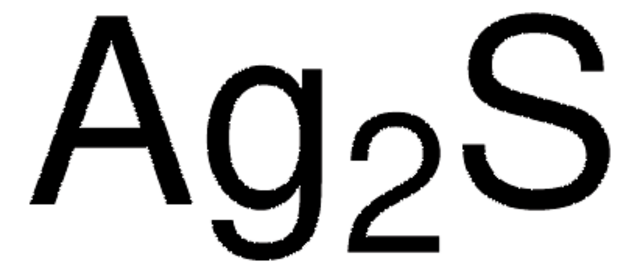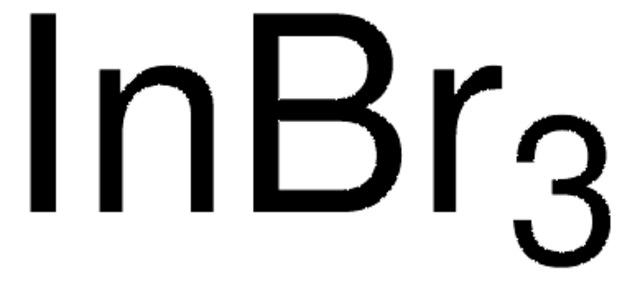추천 제품
Quality Level
분석
99%
양식
powder, crystals or chunks
mp
432 °C (lit.)
density
6.473 g/mL at 25 °C (lit.)
SMILES string
Br[Ag]
InChI
1S/Ag.BrH/h;1H/q+1;/p-1
InChI key
ADZWSOLPGZMUMY-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Lattice defects in silver bromide.
Berry CR.
Physical Review, 82(3), 422-422 (1951)
Xiaojie Zhang et al.
Journal of colloid and interface science, 377(1), 277-283 (2012-04-11)
AgBr/palygorskite composite was prepared by an in situ electrostatic adsorption-deposition-precipitation method and characterized by field emission scanning electron microscope (FE-SEM), X-ray diffraction (XRD), UV-Vis diffuse reflection, and BET surface measurements techniques. The layer negative charge and larger specific surface area
Studies on Aging of Precipitates and Coprecipitation XXXIX. Low Temperature Conductivity of Silver Bromide.
Shapiro I and Kolthoff IM.
J. Chem. Phys., 15, 41-46 (1947)
Silver bromide as a photocatalyst for hydrogen generation from CH3OH/H2O solution.
Kakuta N, et al.
The Journal of Physical Chemistry B, 103(29), 5917-5919 (1999)
Fabien Hubert et al.
Langmuir : the ACS journal of surfaces and colloids, 24(17), 9219-9222 (2008-08-12)
A complex between cetyltrimethylammonium bromide (CTAB) surfactant and silver bromide (CTASB) is recognized by NMR and X-ray photoelectron spectroscopy (XPS) to be the entity at the surface of gold nanorods, resulting from an in situ formation in the classical scheme
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.