Y0000046
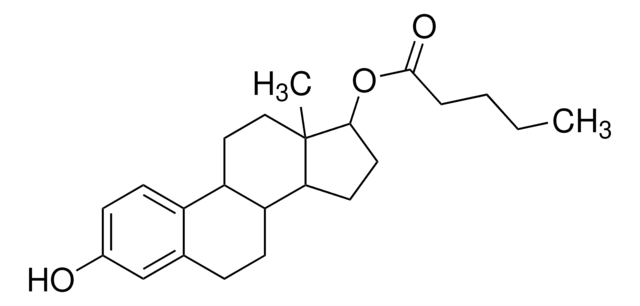
Estradiol valerate
European Pharmacopoeia (EP) Reference Standard
동의어(들):
β-Estradiol 17-valerate, 1,3,5(10)-Estratriene-3,17β-diol 17-pentanote
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C23H32O3
CAS Number:
Molecular Weight:
356.50
Beilstein:
2480357
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
estradiol
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CCCCC(=O)OC1CCC2C3CCc4cc(O)ccc4C3CCC12C
InChI
1S/C23H32O3/c1-3-4-5-22(25)26-21-11-10-20-19-8-6-15-14-16(24)7-9-17(15)18(19)12-13-23(20,21)2/h7,9,14,18-21,24H,3-6,8,10-13H2,1-2H3
InChI key
RSEPBGGWRJCQGY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Estradiol valerate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 1 - Carc. 2 - Lact. - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
A Teichmann
Climacteric : the journal of the International Menopause Society, 6 Suppl 2, 17-23 (2003-12-13)
The particular features of the pharmacology of a new continuous regimen for hormone replacement therapy containing 2 mg estradiol valerate (E2V) and 2 mg dienogest (DNG) (Climodien, Schering AG, Berlin, Germany) depend largely on its progestogenic component. Dienogest has the
C Egarter et al.
Acta obstetricia et gynecologica Scandinavica, 75(4), 386-393 (1996-04-01)
Two sequential hormone replacement regimens, containing either estradiol valerate plus medroxyprogesterone acetate (E2V/MPA) or conjugated estrogens plus medrogestone (CE/MED), were compared with respect to effects on climacteric symptoms, lipid metabolism, and hemostasis. In an open, multicenter study, 51 perimenopausal women
R M Machado-de-Sena et al.
Photodiagnosis and photodynamic therapy, 11(3), 275-282 (2014-05-06)
Vaginal candidiasis (VC) is a disease that affects thousands of women of childbearing age, mainly caused by Candida albicans fungus. Photodynamic therapy (PDT) uses photosensitizing substances that are nontoxic in the dark, but able to produce reactive oxygen species when
Franca Fruzzetti et al.
Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 28(5), 400-408 (2012-04-04)
Natural estrogens such as estradiol (E(2)) or its valerate ester (E(2)V) offer an alternative to ethinyl estradiol (EE). E(2)-containing combined oral contraceptives (COCs) have demonstrated sufficient ovulation inhibition and acceptable contraceptive efficacy. However, earlier formulations were generally associated with unacceptable
Clinical efficacy and safety of combined estradiol valerate and dienogest: a new no-bleed treatment.
B von Schoultz
Climacteric : the journal of the International Menopause Society, 6 Suppl 2, 24-32 (2003-12-13)
A combination of 2 mg estradiol valerate with 2 mg dienogest (E2V/DNG) (Climodien, Schering AG, Berlin, Gemany) is the first continuous combined postmenopausal hormone replacement therapy (HRT) preparation to contain a progestogen with substantial antiandrogenic activity. A study of its
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.