43720
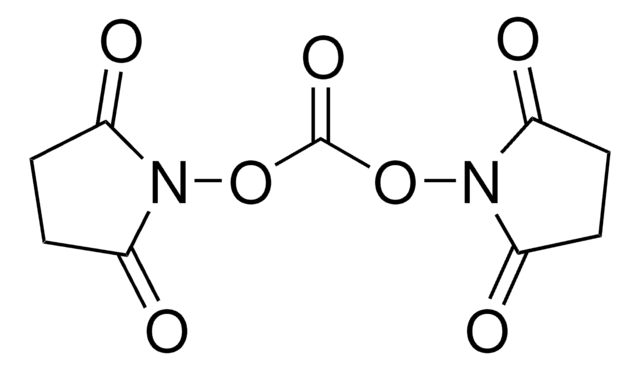
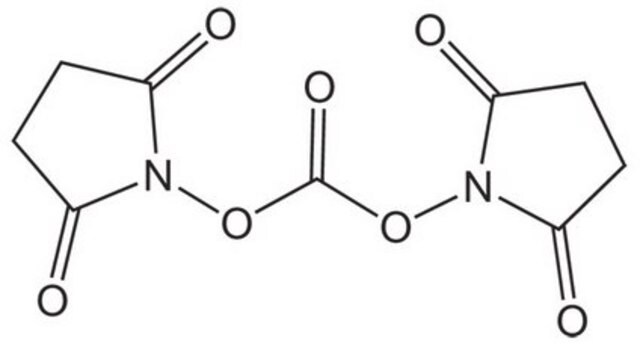
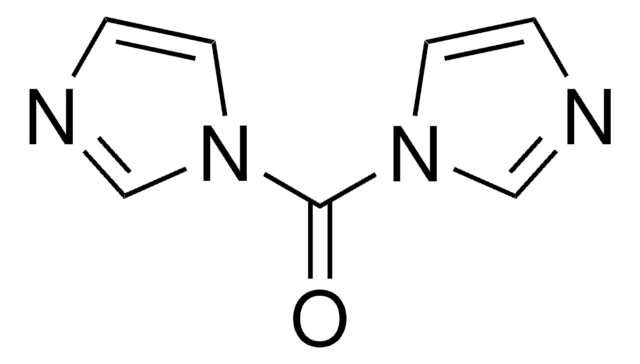
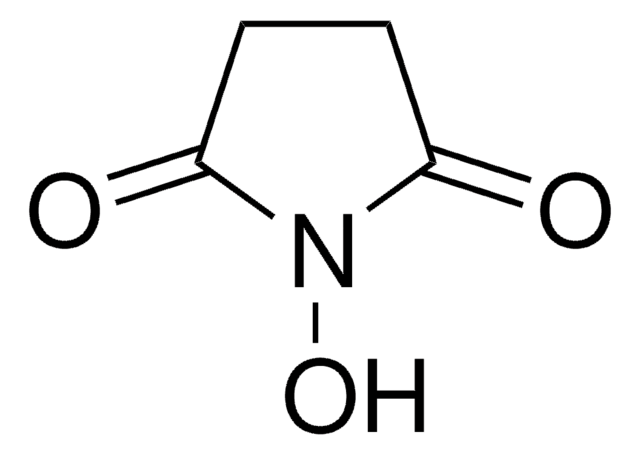
N,N′-Disuccinimidyl carbonate
≥95.0% (NMR), for peptide synthesis
동의어(들):
N-Succinimidyl carbonate, DSC, Di(N-succinimidyl) carbonate
About This Item
추천 제품
제품명
N,N′-Disuccinimidyl carbonate, purum, ≥95.0% (NMR)
grade
purum
Quality Level
분석
≥95.0% (NMR)
양식
powder
반응 적합성
reaction type: Carbonylations
불순물
~3% N-hydroxysuccinimide (NMR)
mp
190 °C (dec.) (lit.)
응용 분야
peptide synthesis
작용기
imide
저장 온도
−20°C
SMILES string
O=C1CCC(=O)N1OC(=O)ON2C(=O)CCC2=O
InChI
1S/C9H8N2O7/c12-5-1-2-6(13)10(5)17-9(16)18-11-7(14)3-4-8(11)15/h1-4H2
InChI key
PFYXSUNOLOJMDX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Various carbamate derivatives from primary and sterically hindered secondary alcohols by alkoxycarbonylation.
- Active carbonate resins from 4-hydroxymethylpolystyrene and 4-hydroxymethyl-3-nitrobenzamido resins via hydroxy functional groups.
- Aza-glycinyl dipeptides, important intermediates for the preparation of various azapeptides.
It may be also used:
- In the two-step preparation of 5-(6-(azidomethyl)nicotinamido)pentanoic acid, a copper-chelating picolyl azide derivative.
- To activate the hydroxyl group of the hapten, γ-hydroxyphenylbutazone (HPBZ) so that HPBZ can effectively bind with human serum albumin(HSA)-immunogen to form a hapten-protein conjugate.
기타 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
문서
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.