추천 제품
Grade
derivatization grade ((chiral))
Quality Level
제품 라인
ChiraSelect™
광학 순도
enantiomeric ratio: ≥99.0:1.0 (HPLC)
품질
LiChropur™
농도
≥18 mM in acetone
기술
HPLC: suitable
refractive index
n20/D 1.359
저장 온도
2-8°C
SMILES string
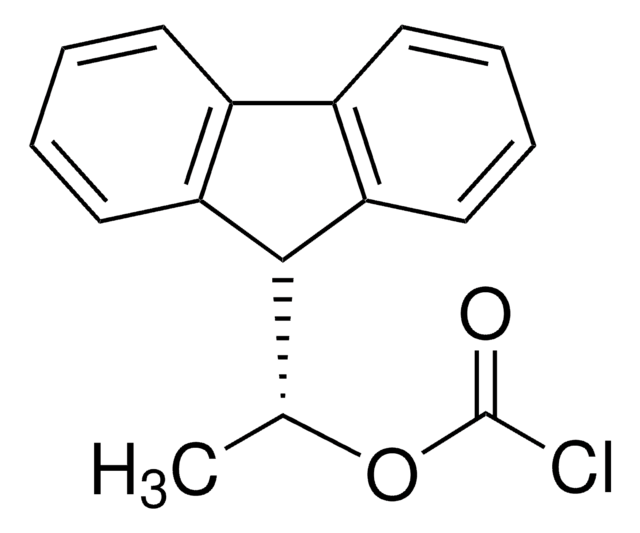
ClC(=O)OC(C1c2c(cccc2)c3c1cccc3)C
InChI
1S/C16H13ClO2/c1-10(19-16(17)18)15-13-8-4-2-6-11(13)12-7-3-5-9-14(12)15/h2-10,15H,1H3
InChI key
SFRVOKMRHPQYGE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(+)-1-(9-Fluorenyl)ethyl chloroformate [(+)-FLEC] is a chiral derivatizing reagent. It helps in separating enantiomers of α-amino acids, as well as helps in determining its optical purity using HPLC method.
애플리케이션
(+)-FLEC may be used in enantioselective analysis of glufosinate using reversed-phase high-performance liquid chromatography system coupled with a fluorescence detection system.
물리적 형태
solution, 5 mg in 1 mL acetone
기타 정보
Chiral derivatizing agent for primary and secondary amino acids and amines. The derivatives are separated by reversed-phase LC with fluorescence detection for determining the enantiomeric purity
법적 정보
ChiraSelect is a trademark of Sigma-Aldrich Co. LLC
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
1.4 °F
Flash Point (°C)
-17 °C
Separation of enantiomers of a-hydroxy acids by reversed-phase liquid chromatography after derivatization with 1-(9-fluorenyl) ethyl chloroformate.
Fransson, Bengt, and Ulf Ragnarsson.
Journal of Chromatography A, 827, 31-36 (1998)
E Frigerio et al.
Journal of chromatography. A, 660(1-2), 351-358 (1994-02-04)
A sensitive and selective high-performance liquid chromatographic method for the determination of reboxetine enantiomers in human plasma was developed. Although two chiral centres are present in reboxetine, its stereospecific synthesis leads to two rather than four possible enantiomers. After extraction
Y Bergqvist et al.
Journal of chromatography, 652(1), 73-81 (1994-01-14)
A sensitive, stereoselective and rapid reversed-phase liquid chromatographic method for the determination of (SR)- and (RS)-mefloquine enantiomers in 100 microliters plasma and capillary blood collected on chromatographic paper is presented. The assay involves protein precipitation from plasma, liquid-liquid extraction of
K C Chan et al.
Electrophoresis, 16(4), 504-509 (1995-04-01)
Direct enantiomeric separations of some racemic amino acids derivatized with 9-fluorenylmethyl chloroformate were obtained using cyclodextrin-modified micellar electrokinetic chromatography (CD/MEKC) with a buffer made up of 5 mM sodium borate (pH 9.2), 150 mM sodium dodecyl sulfate (SDS) and 40
J Sukbuntherng et al.
Journal of analytical toxicology, 19(3), 139-147 (1995-05-01)
To study the disposition kinetics of methamphetamine (MAP), we have developed a sensitive high-performance liquid chromatographic (HPLC) assay to quantitate the enantiomers of MAP and its major metabolites, amphetamine (AP), p-hydroxymethamphetamine (p-OH-MAP), and p-hydroxyamphetamine (p-OH-AP), the latter two of which
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.