추천 제품
Quality Level
분석
98%
mp
100-105 °C (lit.)
SMILES string
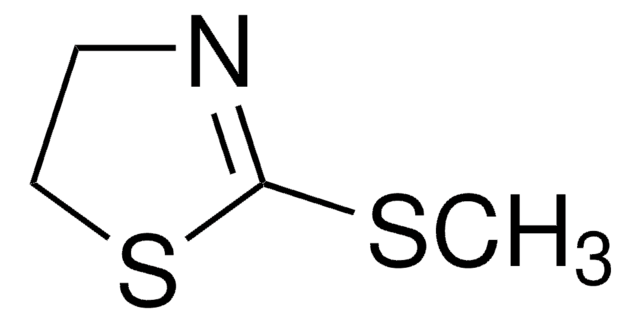
S=C1NCCS1
InChI
1S/C3H5NS2/c5-3-4-1-2-6-3/h1-2H2,(H,4,5)
InChI key
WGJCBBASTRWVJL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Tool for highly selective chiral syntheses of penam- and carbapenam-type β-lactam antibiotics.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Chem. Abstr., 108, 37417r-37417r (1988)
Stud. Org. Chem., 28, 57-57 (1987)
Yahia N Mabkhot et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-01)
A series of new thiazoline derivatives were synthesized. Structure analyses were accomplished employing 1H-NMR, 13C-NMR, X-ray and MS techniques. The in vitro antitumor activities were assessed against human hepatocellular carcinoma (HepG-2) and colorectal carcinoma (HCT-116) cell lines. The results revealed
R B Greenwald et al.
Bioconjugate chemistry, 7(6), 638-641 (1996-11-01)
A novel PEG linker that employs a thiazolidine-2-thione group has been synthesized. Kinetic studies done on this compound demonstrate a relatively long half-life compared to those of traditional succinimidyl linkers. This new PEG derivative reacts with proteins under mild conditions
Denis L Guerra et al.
Journal of hazardous materials, 183(1-3), 81-86 (2010-08-03)
The synthetic imogolite sample was used for organofunctionalization process with 2-mercaptothiazoline (MTZ). The compound 2-mercaptothiazoline was anchored onto imogolite surface by heterogeneous route. Due to the increment of basic centers attached to the pendant chains the dye adsorption capability of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.


![Poly[[6-[(1,1,3,3-tetramethylbutyl)amino]-s-triazine-2,4-diyl]-[(2,2,6,6-tetramethyl-4-piperidyl)imino]-hexamethylene-[(2,2,6,6-tetramethyl-4-piperidyl)imino] average Mn ~2,000](/deepweb/assets/sigmaaldrich/product/structures/679/088/c718a900-edcf-4dfa-ac1e-c410f3f12ab5/640/c718a900-edcf-4dfa-ac1e-c410f3f12ab5.png)