추천 제품
Quality Level
분석
97%
mp
91-93 °C (lit.)
solubility
1 M HCl: soluble 50 mg/mL, clear (dark yellow-brown)
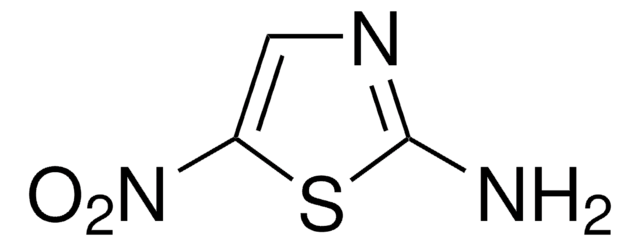
SMILES string
Nc1nccs1
InChI
1S/C3H4N2S/c4-3-5-1-2-6-3/h1-2H,(H2,4,5)
InChI key
RAIPHJJURHTUIC-UHFFFAOYSA-N
애플리케이션
2-Aminothiazole was used in the synthesis of 2-aminothiazole-modified silica gel. It was used in Ulmann coupling with 2-chlorobenzoic acids mediated by ultrasonic irradiation.
생화학적/생리학적 작용
2-Aminothiazoles are potent cyclin-dependent kinase 5 inhibitors and are therapeutic agents for the treatment of Alzheimer′s disease and other neurodegenerative disorders.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Xin Cao et al.
Bioorganic & medicinal chemistry, 16(11), 5890-5898 (2008-05-20)
Because both c-Src and iNOS are key regulatory enzymes in tumorigenesis, a new series of 4-heteroarylamino-3-quinolinecarbonitriles as potent dual inhibitors of both enzymes were designed, prepared, and evaluated for blocking multiple signaling pathways in cancer therapy. All compounds were evaluated
Synthetic Communications, 37, 1853-1853 (2007)
Christopher J Helal et al.
Bioorganic & medicinal chemistry letters, 14(22), 5521-5525 (2004-10-16)
High-throughput screening with cyclin-dependent kinase 5 (cdk5)/p25 led to the discovery of N-(5-isopropyl-thiazol-2-yl)isobutyramide (1). This compound is an equipotent inhibitor of cdk5 and cyclin-dependent kinase 2 (cdk2)/cyclin E (IC(50)=ca. 320nM). Parallel and directed synthesis techniques were utilized to explore the
P S Roldan et al.
Analytical and bioanalytical chemistry, 375(4), 574-577 (2003-03-01)
This work describes the synthesis and characterization of 2-aminothiazole-modified silica gel (SiAT), as well as its application for preconcentration (in batch and column technique) of Cu(II), Ni(II) and Zn(II) in ethanol medium. The adsorption capacities of SiAT determined for each
Ian Bruce et al.
Bioorganic & medicinal chemistry letters, 22(17), 5445-5450 (2012-08-07)
Using a parallel synthesis approach to target a non-conserved region of the PI3K catalytic domain a pan-PI3K inhibitor 1 was elaborated to provide alpha, delta and gamma isoform selective Class I PI3K inhibitors 21, 24, 26 and 27. The compounds
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.