906328
BTTAA
≥95%
동의어(들):
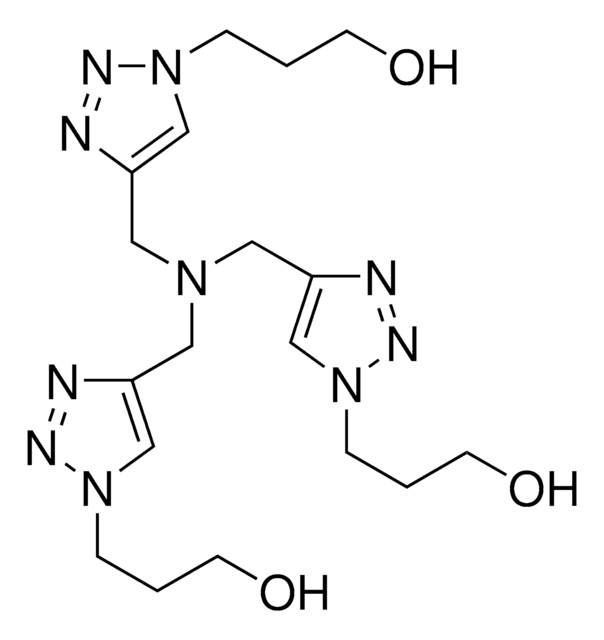
2-(4-((Bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid, Copper click-chemistry ligand, Water-soluble CuAAC ligand
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H30N10O2
CAS Number:
Molecular Weight:
430.51
MDL number:
UNSPSC 코드:
12352200
추천 제품
분석
≥95%
양식
solid
반응 적합성
reaction type: click chemistry
재고 정보
available only in USA
저장 온도
2-8°C
SMILES string
[n]1(nnc(c1)CN(Cc3nn[n](c3)C(C)(C)C)Cc2nn[n](c2)CC(=O)O)C(C)(C)C
InChI
1S/C19H30N10O2/c1-18(2,3)28-11-15(21-24-28)8-26(7-14-10-27(23-20-14)13-17(30)31)9-16-12-29(25-22-16)19(4,5)6/h10-12H,7-9,13H2,1-6H3,(H,30,31)
InChI key
MGQYHUDOWOGSQI-UHFFFAOYSA-N
애플리케이션
BTTAA is a next-generation, water-soluble ligand for the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) that dramatically accelerates reaction rates and suppresses cell cytotoxicity. The biocompatibility and fast kinetics of BTTAA are advancements from water-insoluble TBTA and are desirable for bio conjugation in diverse chemical biology experiments.
기타 정보
Biocompatible click chemistry enabled compartment-specific pH measurement inside E. coli
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling.
Chayasith Uttamapinant et al.
Angewandte Chemie (International ed. in English), 51(24), 5852-5856 (2012-05-05)
Samra Obeid et al.
Chemical communications (Cambridge, England), 48(67), 8320-8322 (2012-07-07)
Modified nucleotides play a paramount role in many cutting-edge biomolecular techniques. The present structural study highlights the plasticity and flexibility of the active site of a DNA polymerase while incorporating non-polar "Click-able" nucleotide analogs and emphasizes new insights into rational
Christen Besanceney-Webler et al.
Bioorganic & medicinal chemistry letters, 21(17), 4989-4992 (2011-06-17)
Members of the Bacteroidales order are among the most abundant gram-negative bacteria of the human colonic microbiota. These species decorate their cell-surface glycoproteins with fucosylated glycans, which are believed to play important roles in host intestinal colonization. Currently, there is
Junfeng Chen et al.
Journal of the American Chemical Society, 140(42), 13695-13702 (2018-09-08)
A major challenge in performing reactions in biological systems is the requirement for low substrate concentrations, often in the micromolar range. We report that copper cross-linked single-chain nanoparticles (SCNPs) are able to significantly increase the efficiency of copper(I)-catalyzed alkyne-azide cycloaddition
Yong Liang et al.
Analytical chemistry, 86(8), 3688-3692 (2014-03-25)
P450 3A4 (CYP3A4) is one of the most important isoforms in the human cytochrome P450 superfamily. It was used as an example in this proof-of-concept study in order to demonstrate an activity-based labeling and then click chemistry (CC) mediated element-tagging
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)