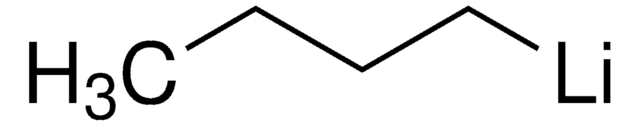
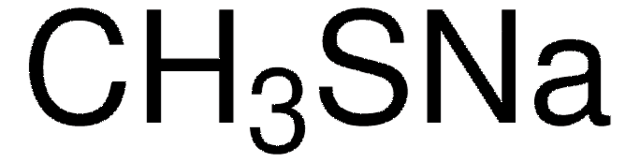추천 제품
Quality Level
분석
0.79 M in THF
양식
liquid
농도
0.7-0.9 M in THF
적합성
conforms to structure for Proton NMR spectrum
저장 온도
2-8°C
SMILES string
S
InChI
1S/H2S/h1H2
InChI key
RWSOTUBLDIXVET-UHFFFAOYSA-N
애플리케이션
Hydrogen sulfide solution can be used in the preparation of lead sulfide (PbS), titanium disulfide(TiS2) and cadmium sulfide (CdS). It is also used as a ligand in the synthesis of ammineruthenium complexes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup - Solvent
Flash Point (°C)
-17 °C - closed cup - Solvent
Modified Sol-Gel Processing for Titanium Disulfide.
Go, Yong Bok et al.
J. Korean Chem. Soc., 41(3), 130-137 (1997)
Cadmium sulphide quantum dots in morphologically tunable triblock copolymer aggregates.
Duxin, Nicolas et al.
Journal of the American Chemical Society, 127(28), 10063-10069 (2005)
Size variation of PbS particles in high-refractive-index nanocomposites.
Kyprianidou-Leodidou, Tasoula et al.
The Journal of Physical Chemistry, 98(36), 8992-8997 (1994)
Amine Ruthenium complexes of hydrogen sulfide and related sulfur ligands.
Kuehn CC
Journal of the American Chemical Society, 98(3), 689-702 (1976)
Vivian S Lin et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(18), 7131-7135 (2013-04-17)
Hydrogen sulfide (H2S) is a reactive small molecule generated in the body that can be beneficial or toxic owing to its potent redox activity. In living systems, disentangling the pathways responsible for H2S production and their physiological and pathological consequences
프로토콜
Separation of Sulfur dioxide; Hydrogen sulfide; Carbonyl sulfide; Methanethiol; Ethanethiol; Dimethyl disulfide; Carbon disulfide
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.