모든 사진(1)
About This Item
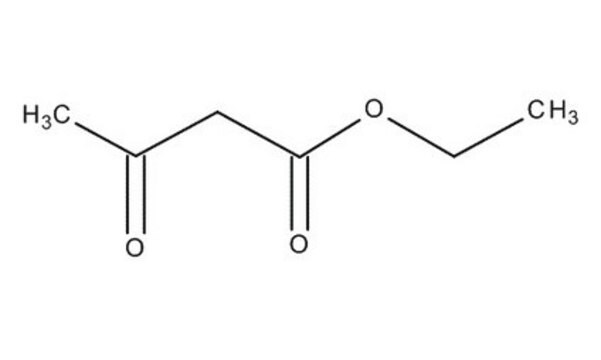
Linear Formula:
CH3COCH2COOC2H5
CAS Number:
Molecular Weight:
130.14
Beilstein:
385838
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor density
4.48 (vs air)
Quality Level
vapor pressure
1 mmHg ( 28.5 °C)
분석
≥99.0% (GC)
양식
liquid
autoignition temp.
580 °F
품질
Arxada quality
expl. lim.
9.5 %
제조업체/상표
Arxada AG
불순물
≤0.05% water
≤0.10% acid (as acetic acid)
색상
APHA: ≤15
bp
180 °C
181 °C (lit.)
mp
−43 °C (lit.)
solubility
water: soluble 130 g/L at 20 °C
density
1.029 g/mL at 20 °C (lit.)
SMILES string
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Ethyl acetoacetate can undergo:
- Transesterification with primary and secondary alcohols in the presence of boric acid.
- Microbial reduction to form (S)-ethyl 3-hydroxybutyrate.
- C-acylation by acid chlorides in the presence of magnesium chloride and pyridine.
- Asymmetric biphasic catalytic hydrogenation in the presence of a water-soluble ruthenium-BINAP complex.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
164.3 °F - closed cup
Flash Point (°C)
73.5 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Hydrogenation of ethyl acetoacetate catalyzed by hydrosoluble BINAP derivatives
Lamouille T, et al.
Tetrahedron Letters, 42(4), 663-664 (2001)
Production of (+)-(S)-Ethyl 3-Hydroxybutyrate and (−)-(R)-Ethyl 3-Hydroxybutyrate by Microbial Reduction of Ethyl Acetoacetate
Wipf B, et al.
Helvetica Chimica Acta, 66(2), 485-488 (1983)
Enantioselective catalytic asymmetric hydrogenation of ethyl acetoacetate in room temperature ionic liquids
Berthod M, et al.
Tetrahedron Asymmetry, 15(14), 2219-2221 (2004)
Boric acid: an efficient and environmentally benign catalyst for transesterification of ethyl acetoacetate
Kondaiah GCM, et al.
Tetrahedron Letters, 49(1), 106-109 (2008)
Procedures for the acylation of diethyl malonate and ethyl acetoacetate with acid chlorides using tertiary amine bases and magnesium chloride.
Rathke MW and Cowan PJ.
The Journal of Organic Chemistry, 50(15), 2622-2624 (1985)
문서
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.