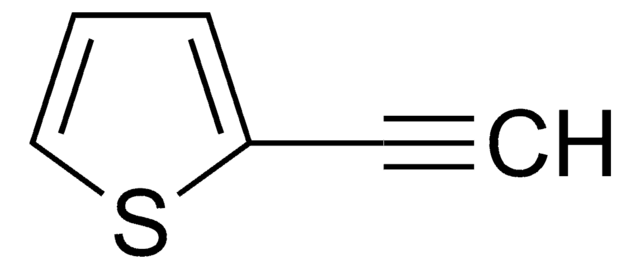
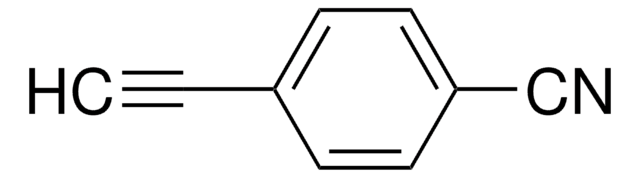
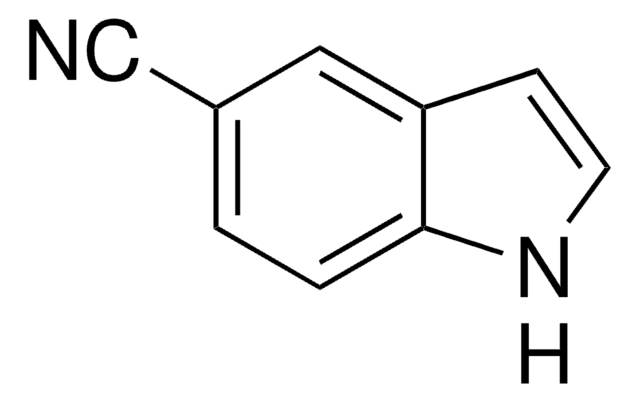
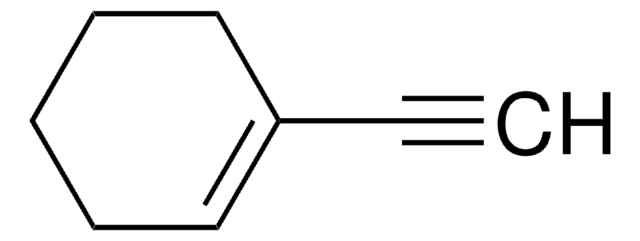
추천 제품
Quality Level
분석
98%
bp
83-84 °C/30 mmHg (lit.)
mp
39-40 °C (lit.)
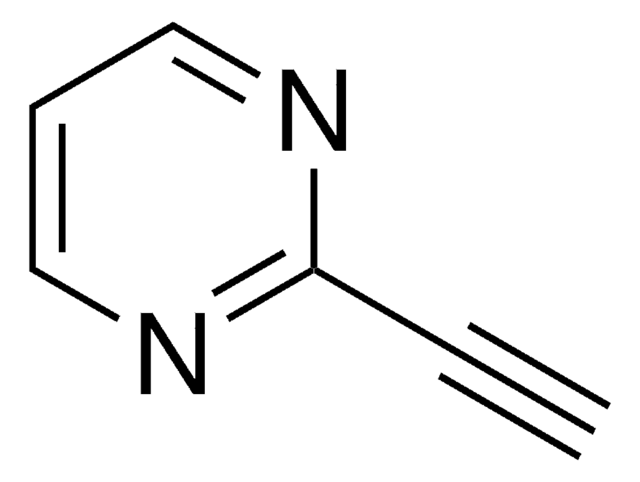
SMILES string
C#Cc1cccnc1
InChI
1S/C7H5N/c1-2-7-4-3-5-8-6-7/h1,3-6H
InChI key
CLRPXACRDTXENY-UHFFFAOYSA-N
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point (°F)
134.0 °F - closed cup
Flash Point (°C)
56.67 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Varinder K Aggarwal et al.
The Journal of organic chemistry, 68(13), 5381-5383 (2003-06-21)
A convenient one-pot procedure for the preparation of pyrazoles by 1,3-dipolar cycloaddition of diazo compounds generated in situ has been developed. Diazo compounds derived from aldehydes were reacted with terminal alkynes to furnish regioselectively 3,5-disubstituted pyrazoles. Furthermore, the reaction of
Wei Wei et al.
Chemical communications (Cambridge, England), 48(2), 305-307 (2011-11-18)
The first transition-metal-catalyzed direct oxidative synthesis of amides by using dioxygen as an oxygen source has been developed under mild conditions, in which DBU was used as the key additive. The present methodology, which utilizes dioxygen as an oxidant and
Christoph Gütz et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(33), 10890-10894 (2013-07-05)
A 1,1'-binaphthyl-based bis(pyridine) ligand (1) was prepared in racemic and enantiomerically pure form to study the formation of [Pd2(1)4] complexes upon coordination to palladium(II) ions with regard to the degree of chiral self-sorting. The self-assembly process proceeds in a highly
Qing Li et al.
Marine drugs, 16(4) (2018-03-31)
Chitosan is an abundant and renewable polysaccharide, which exhibits attractive bioactivities and natural properties. Improvement such as chemical modification of chitosan is often performed for its potential of providing high bioactivity and good water solubility. A new class of chitosan
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
문서
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.






![3-[(Trimethylsilyl)ethynyl]pyridine 97%](/deepweb/assets/sigmaaldrich/product/structures/343/531/3049f5ac-7c3c-45ca-b43c-809abc2f3c9d/640/3049f5ac-7c3c-45ca-b43c-809abc2f3c9d.png)