245801
N-(Hydroxymethyl)acrylamide solution
48 wt. % in H2O
동의어(들):
Monomethylolacrylamide, N-(Hydroxymethyl)acrylamide, N-Methanolacrylamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
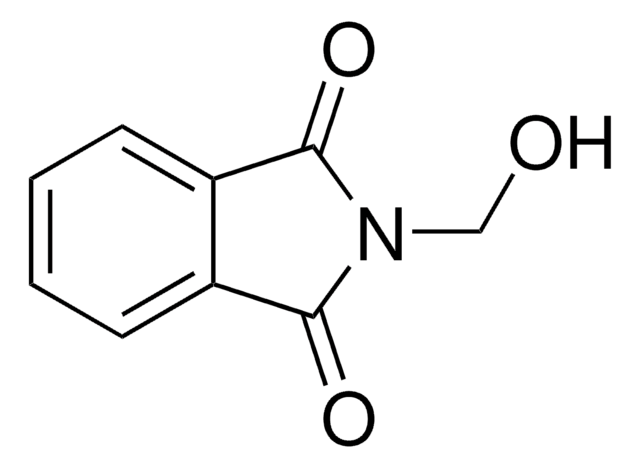
CH2=CHCONHCH2OH
CAS Number:
Molecular Weight:
101.10
Beilstein:
506646
MDL number:
UNSPSC 코드:
12162002
PubChem Substance ID:
NACRES:
NA.23
추천 제품
양식
liquid
Quality Level
포함
30 ppm monomethyl ether hydroquinone as inhibitor
농도
48 wt. % in H2O
refractive index
n20/D 1.413
density
1.074 g/mL at 25 °C
SMILES string
OCNC(=O)C=C
InChI
1S/C4H7NO2/c1-2-4(7)5-3-6/h2,6H,1,3H2,(H,5,7)
InChI key
CNCOEDDPFOAUMB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N-(Hydroxymethyl)acrylamide (NHMA) is a bifunctional monomer with both a reactive vinyl group and a hydroxymethyl group, making it significant in polymer chemistry. It is known for forming hydrophilic, cross-linked networks, with applications in biomedical fields such as drug delivery systems, contact lenses, and tissue engineering, as well as in industrial UV-curable resins. NHMA is commonly used in hydrogel formulations for drug delivery and wound dressings. Additionally, it can be applied in creating bioadhesives or sealants for medical purposes, such as sealing wounds or attaching medical devices to tissues.
애플리케이션
N-(Hydroxymethyl)acrylamide can be used as:
- A monomer in the development of polymer gel dosimeters specifically designed for radiation therapy applications. The incorporation of NHMA enhances the sensitivity of the dosimeter to radiation doses.
- A monomer in the development of thermo-responsive polymeric gates, which exhibits thermo-responsive behavior. The incorporation of NHMA enhances the overall properties of the polymer, allowing for better control over the release mechanisms in response to temperature changes.
- A key monomer in the synthesis of double-hydrophilic and amphiphilic block glycopolymers, contributing to their self-assembly properties, biocompatibility, and functionalization potential. These characteristics make NHMA-based glycopolymers valuable for various biomedical applications, including drug delivery systems, biosensors, and tissue engineering.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Carc. 1B - Muta. 1B - Repr. 2 - Skin Sens. 1 - STOT RE 1 - STOT RE 2 Oral
표적 기관
Peripheral nervous system
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Lars Ole Goffeng et al.
Neurotoxicology and teratology, 30(3), 186-194 (2008-03-21)
The study examines possible persisting effects on the peripheral nervous system and visual system in tunnel workers previously exposed to acrylamide and N-methylolacrylamide during grouting work. We compared neurophysiological function in 44 tunnel workers previously exposed during grouting operations (2-10
Timothy R Fennell et al.
Toxicological sciences : an official journal of the Society of Toxicology, 71(2), 164-175 (2003-02-04)
Acrylamide (AM) and N-methylolacrylamide (NMA) are used in the formulation of grouting materials. AM undergoes metabolism to a reactive epoxide, glycidamide (GA). Both AM and GA react with hemoglobin to form adducts that can be related to exposure to AM.
Helge Kjuus et al.
Scandinavian journal of work, environment & health, 30(1), 21-29 (2004-03-17)
This study evaluates the possible toxic effects on the peripheral nervous system of tunnel workers exposed to acrylamide and N-methylolacrylamide during grouting work. Symptoms and nerve conduction velocities (NCV) were recorded for 24 tunnel workers 4 and 16 months after
Kristine L Witt et al.
Environmental and molecular mutagenesis, 41(2), 111-120 (2003-02-28)
N-Hydroxymethylacrylamide (NHMA), a mouse carcinogen inactive in the Salmonella assay and mouse micronucleus (MN) assay, was tested for reproductive effects in a mouse continuous breeding study. In that study, increased embryonic deaths were observed after 13 weeks exposure of parental
H Tanii et al.
Toxicology letters, 58(2), 209-213 (1991-10-01)
Acrylamide and 6 derivatives inhibited neurite growth from rat dorsal root ganglion in culture. The half-maximum inhibition concentration (I50) varied among test compounds, ranging from 0.8 mM for acrylamide and N-hydroxymethylacrylamide to 30 mM for methacrylamide. The value correlated well
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.


![N-[Tris(hydroxymethyl)methyl]acrylamide contains ≤7% KCl, 93%](/deepweb/assets/sigmaaldrich/product/structures/130/961/5bc6d1a4-a540-4496-9f46-74507af67e21/640/5bc6d1a4-a540-4496-9f46-74507af67e21.png)