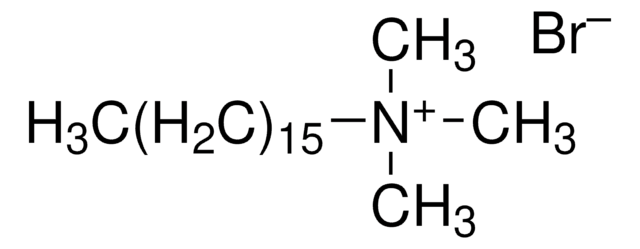모든 사진(1)
About This Item
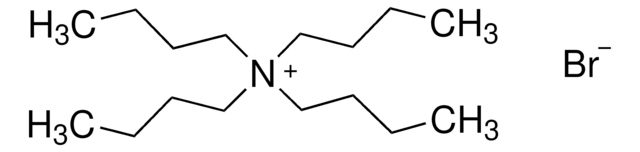
Linear Formula:
[CH3(CH2)4]4N(Br)
CAS Number:
Molecular Weight:
378.47
Beilstein:
3658171
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99%
mp
100-101 °C (lit.)
SMILES string
[Br-].CCCCC[N+](CCCCC)(CCCCC)CCCCC
InChI
1S/C20H44N.BrH/c1-5-9-13-17-21(18-14-10-6-2,19-15-11-7-3)20-16-12-8-4;/h5-20H2,1-4H3;1H/q+1;/p-1
InChI key
SPALIFXDWQTXKS-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Tetrapentylammonium bromide is a quaternary ammonium salt with pentyl chains and a bromide counterion, which is generally used as a phase transfer catalyst.
애플리케이션
Tetrapentylammonium bromide can be used:
Electrochemical synthesis of stable graphite intercalation compounds (GICs) containing tetrapentylammonium cations has been reported.
- As a versatile structure-directing agent for the synthesis of Zeolite-like heterobimetallic cyanide frameworks.
- As an alkylating agent for rhodium(I)-catalyzed alkylation reaction of benzylic amines and for N-alkylation of azaheterocycles.
- As a precursor to prepare a Cobalt-complex, {[(Pentyl)4N]3CoBr3}Cl2, which is used as a catalyst in the synthesis of multiwalled carbon nanotubes (MWCNTs).
Electrochemical synthesis of stable graphite intercalation compounds (GICs) containing tetrapentylammonium cations has been reported.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Quaternary Ammonium Salts as Alkylating Reagents in C-H Activation Chemistry.
Spettel M, et al.
Organic Letters, 19(16), 4287-4290 (2017)
Chemical modification of polymers via phase transfer catalysis
Nishikubo T.
Handbook of Phase Transfer Catalysis, 480-509 (1997)
Novel catalyst based on Co-complex to prepare MWCNT.
Alonso-Nu?ez G, et al.
Materials Letters, 109, 163-166 (2013)
Quaternary ammonium salts as highly efficient and green alkylating agents for N-alkylation of azaheterocycles under microwave irradiation.
Khalafi-Nezhad A, et al.
Journal of the Iranian Chemical Society, 5(1), S40-S46 (2008)
The electrochemical synthesis of the graphite intercalation compounds containing tetra-n-alkylammonium cations.
Sirisaksoontorn W and Lerner MM.
Journal of Sol-Gel Science and Technology, 2(9), M28-M32 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.