227439
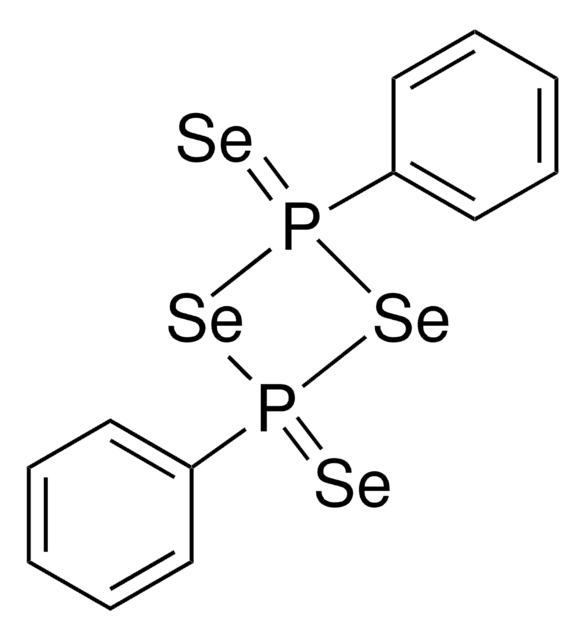
Lawesson reagent
97%
동의어(들):
2,4-Bis(4-methoxyphenyl)-2,4-dithioxo-1,3,2,4-dithiadiphosphetane, 2,4-Bis-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide, 4-Methoxyphenylthiophosphoric cyclic di(thioanhydride), LR
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C14H14O2P2S4
CAS Number:
Molecular Weight:
404.47
Beilstein:
1024888
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
powder
mp
228-230 °C (lit.)
SMILES string
COc1ccc(cc1)P2(=S)SP(=S)(S2)c3ccc(OC)cc3
InChI
1S/C14H14O2P2S4/c1-15-11-3-7-13(8-4-11)17(19)21-18(20,22-17)14-9-5-12(16-2)6-10-14/h3-10H,1-2H3
InChI key
CFHGBZLNZZVTAY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Lawesson′s reagent is generally used as a thiation agent in organic synthesis for the conversion of oxygen functionalities into their thio analogs. It facilitates the conversion of the carbonyl group to thiocarbonyl group as well as carbon-oxygen single bond into a carbon-sulfur single bond.
애플리케이션
Lawesson reagent can be used as a reagent to synthesize:
- Oxthiaphosphinine-3-sulfide derivatives by the reaction with Mannich bases of β-naphthol and 8-hydroxyquinoline.
- 1,3,5,2-Trithiaphosphinane-2-sulfide derivatives by reacting with benzaldehyde in the presence of trialkyl phosphite.
- 2,4,6-Triphenyl-1,3,5-trithiane from benzaldehyde and ethyl acrylate.
- 9-Benzanthronethione by thionation of 9-benzanthone oxime.
- 1,2,4-Trithiolane from 2,2,4,4-tetramethyl-3-thioxocyclobutanone S-oxide.
- Sulfur derivatives of triterpenic oxo compounds.
- Tropothione in situ at room temperature and to trap it with dieneophiles.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Water-react 2
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Qiuping Ding et al.
Journal of combinatorial chemistry, 11(6), 1047-1049 (2009-10-15)
In the presence of Lawesson's reagent, metal-free one-pot cascade reactions of 2-iodoanilines with acid chlorides proceeded smoothly leading to 2-substituted benzothiazoles in good to excellent yields under mild conditions. Three steps were involved in the reaction process: (1) 2-iodoanilines reacted
B G Bruinsma et al.
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 14(6), 1400-1409 (2014-04-25)
To reduce widespread shortages, attempts are made to use more marginal livers for transplantation. Many of these grafts are discarded for fear of inferior survival rates or biliary complications. Recent advances in organ preservation have shown that ex vivo subnormothermic
K A Jørgensen et al.
IARC scientific publications, (41)(41), 159-168 (1982-01-01)
The reaction between N-nitrosamides and 2,4-bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide (LR) at low temperature (20-50 degrees C) gives the corresponding thioamides as the main products. In the reaction between N-nitroso-2-pyrrolidone and LR, dihydro-2-(3H)-thiophenone (III) is also isolated. Mechanistic considerations for the formation of III
Synthesis of 1, 2, 4-trithiolanes from thione S-oxides and Lawesson reagent at room temperature
Okuma K, et al.
Bulletin of the Chemical Society of Japan, 77(1), 187-188 (2004)
A novel protocol for the generation of tropothione and its trapping with electron deficient dienophiles
Nair V, et al.
Tetrahedron Letters, 47(52), 9329-9329 (2006)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.