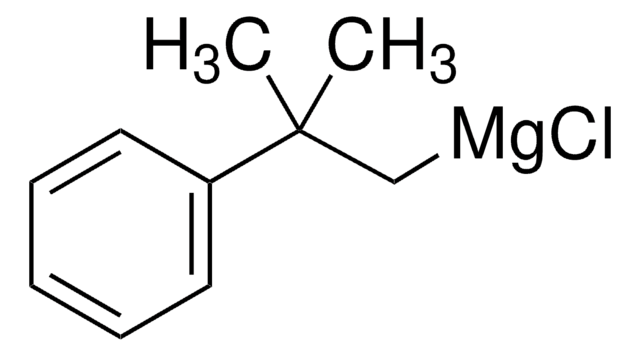
225916
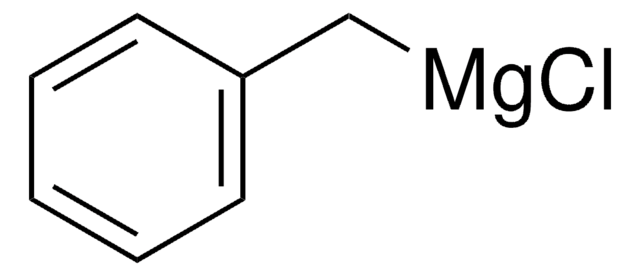
Benzylmagnesium chloride solution
2.0 M in THF
동의어(들):
(Phenylmethyl)magnesium chloride, Benzylchloromagnesium, Chloro(phenylmethyl)magnesium
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5CH2MgCl
CAS Number:
Molecular Weight:
150.89
Beilstein:
878506
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
반응 적합성
reaction type: Grignard Reaction
농도
2.0 M in THF
density
1.031 g/mL at 25 °C
작용기
phenyl
SMILES string
Cl[Mg]Cc1ccccc1
InChI
1S/C7H7.ClH.Mg/c1-7-5-3-2-4-6-7;;/h2-6H,1H2;1H;/q;;+1/p-1
InChI key
NRAFPLGJPPJUNB-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Benzylmagnesium chloride is an organometallic compound that acts as a Lewis acid and a strong base.
애플리케이션
Benzylmagnesium chloride (Grignard reagent) can be used as an alkylating reagent:
- For alkylating quinolyl-functionalized Cp−chromium(III) complexes which are used as precursors for the preparation of active olefin polymerization catalysts.
- In the synthesis of 2,3-disubstituted benzopyran-4-ones, 6-chloro-8-methylpurine derivative, and phenylalanine derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Dichotomy in regioselective cross-coupling reactions of 6, 8-dichloropurines with phenylboronic acid and methylmagnesium chloride: synthesis of 6, 8-disubstituted purines
Hocek M, et al.
Synthesis, 2004(06), 889-894 (2004)
Quinolyl-functionalised Cp-chromium polymerisation catalysts: synthesis and crystal structures of alkylation products
Enders M, et al.
Journal of Organometallic Chemistry, 687(1), 125-130 (2003)
A novel synthesis of 2, 3-disubstituted benzopyran-4-ones and application to the solid phase
Harikrishnan LS and Showalter HD H
Tetrahedron, 56(4), 515-519 (2000)
A facile three-step synthesis of 1, 2-amino alcohols using the Ellman homochiral tert-butylsulfinamide
Barrow JC, et al.
Tetrahedron Letters, 42(11), 2051-2054 (2001)
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.