모든 사진(3)
About This Item
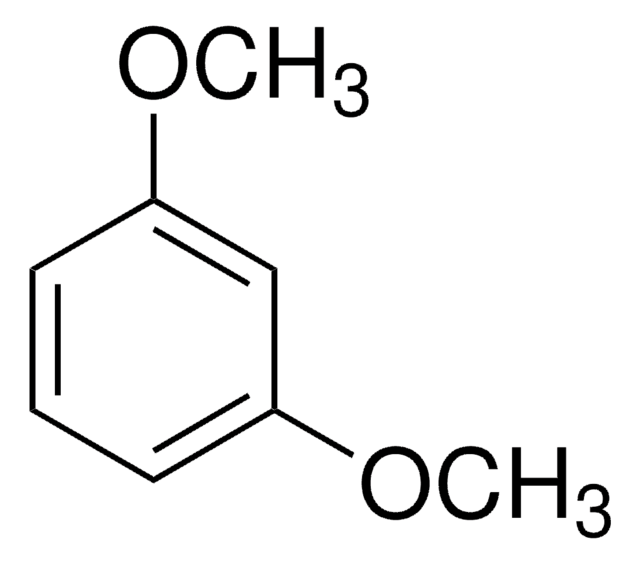
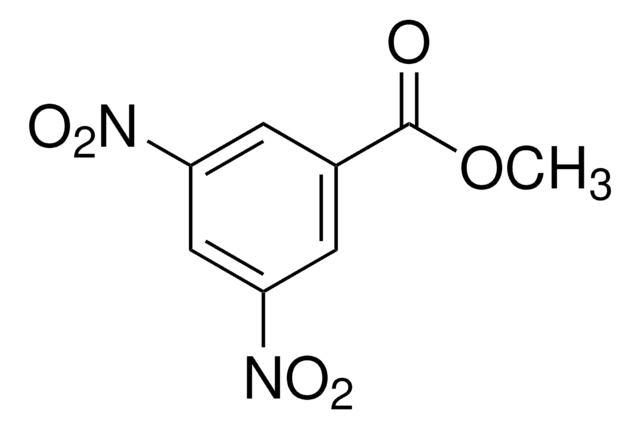
Linear Formula:
C6H4(OCH3)2
CAS Number:
Molecular Weight:
138.16
Beilstein:
878582
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥98%
양식
liquid
refractive index
n20/D 1.524 (lit.)
bp
85-87 °C/7 mmHg (lit.)
density
1.055 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(OC)c1
InChI
1S/C8H10O2/c1-9-7-4-3-5-8(6-7)10-2/h3-6H,1-2H3
InChI key
DPZNOMCNRMUKPS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
1,3-Dimethoxybenzene has been used in the synthesis of novel oxathiane spiroketal donors. It forms pi- and O-ylidic complexes with dichlorocarbene (CCl(2)).
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
190.4 °F - closed cup
Flash Point (°C)
88 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Helena Zahradnícková et al.
Journal of separation science, 29(2), 236-241 (2006-03-10)
For the first time, headspace solid-phase microextraction coupled with GC-MS analysis was used to study volatile compounds emitted by the tick Ixodes ricinus (L.). Variables such as the type of SPME fibre, equilibration time and extraction time have been evaluated
Panagiotis Stathopoulos et al.
Journal of peptide science : an official publication of the European Peptide Society, 12(3), 227-232 (2005-08-17)
Decomposition of the resin linkers during TFA cleavage of the peptides in the Fmoc strategy leads to alkylation of sensitive amino acids. The C-terminal amide alkylation, reported for the first time, is shown to be a major problem in peptide
Takashi Iijima et al.
Bioscience, biotechnology, and biochemistry, 73(11), 2547-2548 (2009-11-10)
An efficient synthesis of tri-O-methylated resveratrol is presented using an advanced Heck reaction promoted by Pd(dba)(2) in the presence of P(t-Bu)(3).
Martin A Fascione et al.
Carbohydrate research, 348, 6-13 (2011-12-28)
Novel oxathiane spiroketal donors have been synthesised and activated via an umpolung S-arylation strategy using 1,3,5-trimethoxybenzene and 1,3-dimethoxybenzene. The comparative reactivity of the resulting 2,4,6-trimethoxyphenyl (TMP)- and 2,4-dimethoxyphenyl (DMP)-oxathiane spiroketal sulfonium ions is discussed, and their α-stereoselectivity in glycosylation reactions
Decrease in glucose oxidation in isolated brown fat cells from rats due to tropolone and dimethoxybenzene.
J W Rosenthal
General pharmacology, 12(1), 47-50 (1981-01-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.