112402
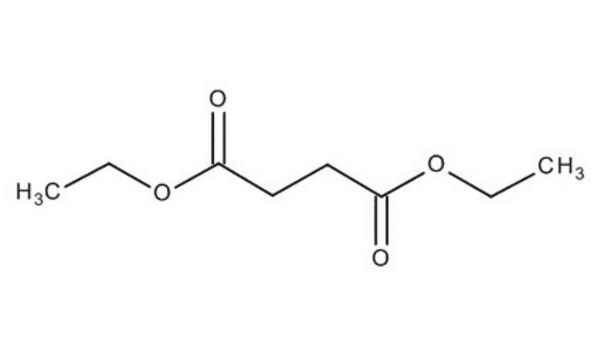
Diethyl succinate
ReagentPlus®, 99%
동의어(들):
1,4-Diethyl butanedioate, Diethyl butanedioate, Ethyl succinate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C2H5OCOCH2CH2COOC2H5
CAS Number:
Molecular Weight:
174.19
Beilstein:
907645
EC Number:
MDL number:
UNSPSC 코드:
12162002
eCl@ss:
39022839
PubChem Substance ID:
NACRES:
NA.23
추천 제품
vapor density
6 (vs air)
Quality Level
제품 라인
ReagentPlus®
분석
99%
양식
liquid
refractive index
n20/D 1.42 (lit.)
bp
218 °C (lit.)
mp
−20 °C (lit.)
density
1.047 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CCC(=O)OCC
InChI
1S/C8H14O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-6H2,1-2H3
InChI key
DKMROQRQHGEIOW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Diethyl succinate(DES) is a diethyl ester with succinate molecules. It has two ester groups and is majorly used in fragrances. It produced by the esterification of succinic acid with ethanol.
애플리케이션
DES and 1-octanol can be blended with B5 palm oil biodiesel to improve the oxygen content and achieve a greener emission of combustion gases. It may also be used as a novel and highly efficient solvent to capture carbon dioxide(CO2) which can be potentially used as a technique to reduce carbon emission.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point (°F)
208.4 °F - closed cup
Flash Point (°C)
98 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Performance and emission characteristics of green diesel blends containing diethyl-succinate and 1-octanol.
Phoon LY, et al.
Journal of Cleaner Production, 161, 1192-1202 (2017)
Diethyl succinate synthesis by reactive distillation
Orjuela A, et al.
Separation and Purification Technology, 88, 151-162 (2012)
Performance evaluation of CO2 capture with diethyl succinate.
Li H, et al.
Applied Energy, 200, 119-131 (2017)
M B Ashour et al.
Toxicology and applied pharmacology, 89(3), 361-369 (1987-07-01)
Treatment with 0.5% (w/w) dietary clofibrate, a peroxisome proliferator, for 14 days induced microsomal carboxylesterase activities for five substrates including malathion, clofibrate, diethylsuccinate, diethylphthalate, and p-nitrophenylacetate in liver and kidney of male Swiss-Webster mice and Sprague-Dawley rats. The induction was
Niki M Zacharias et al.
Journal of the American Chemical Society, 134(2), 934-943 (2011-12-08)
The Krebs tricarboxylic acid cycle (TCA) is central to metabolic energy production and is known to be altered in many disease states. Real-time molecular imaging of the TCA cycle in vivo will be important in understanding the metabolic basis of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.