1491004
USP
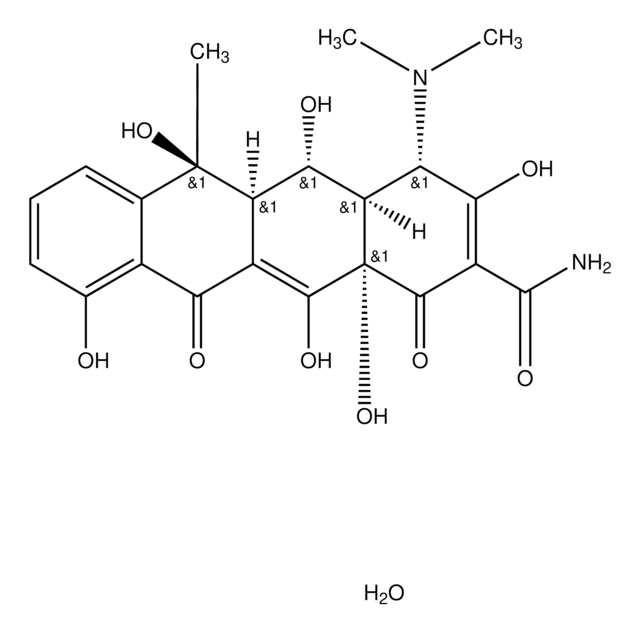
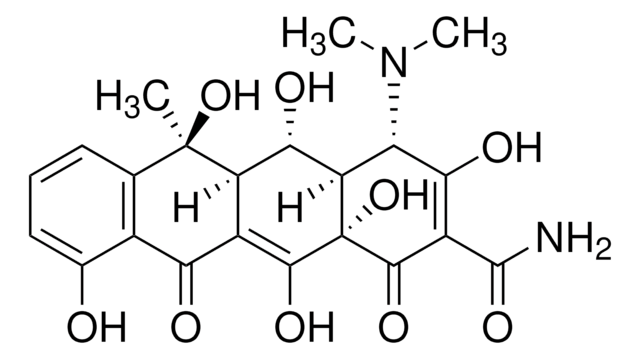
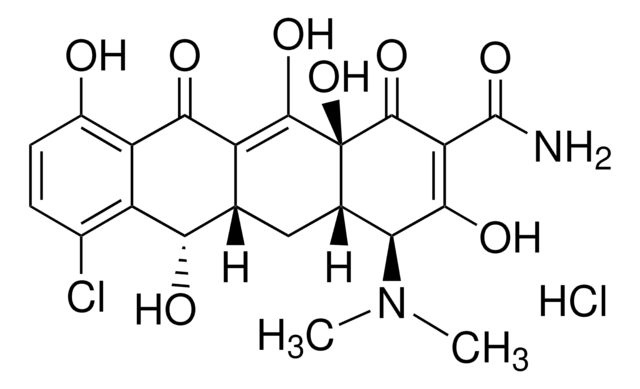
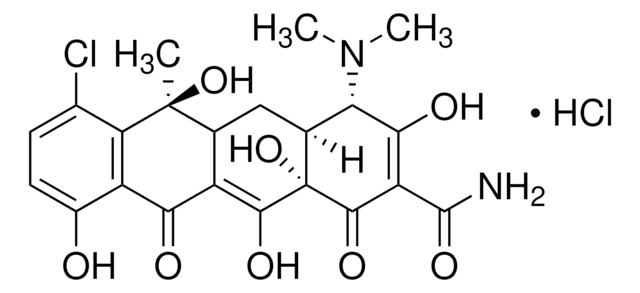
Oxytetracycline
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Oxytetracycline dihydrate
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
oxytetracycline
Forme
solid
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
O.[H][C@@]12[C@@H](O)[C@@]3([H])C(C(=O)c4c(O)cccc4[C@@]3(C)O)=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C
InChI
1S/C22H24N2O9.H2O/c1-21(32)7-5-4-6-8(25)9(7)15(26)10-12(21)17(28)13-14(24(2)3)16(27)11(20(23)31)19(30)22(13,33)18(10)29;/h4-6,12-14,17,25,27-29,32-33H,1-3H3,(H2,23,31);1H2/t12-,13-,14+,17+,21-,22+;/m1./s1
Clé InChI
IBZHEBHGZFICKS-IFLJXUKPSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Oxytetracycline is a broad-spectrum tetracycline antibiotic that inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. It is effective against a range of Gram-positive and Gram-negative organisms. The USP reference standard ensures assay precision and identity confirmation in oxytetracycline drug products.
The USP biologics antibiotics category includes a wide range of antimicrobial agents that are essential in treating bacterial infections. These antibiotics are derived from various natural sources or synthesized to combat specific pathogens effectively. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of antibiotic therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Application
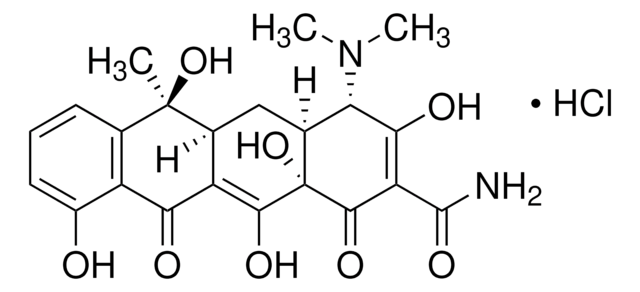
- Oxytetracycline Hydrochloride
- Oxytetracycline Injection
- Oxytetracycline Tablets
- Oxytetracycline for Injection
- Oxytetracycline Hydrochloride Capsules
- Oxytetracycline Hydrochloride Soluble Powder
Actions biochimiques/physiologiques
Mode of Action: Inhibits protein synthesis (elongation) by preventing binding of aminoacyl-tRNA to the 30S subunit.
Antimicrobial spectrum: Gram-negative and Gram-positive bacteria.
Mode of Resistance: Active efflux, ribosome protection, tetracycline inactivation.
Remarque sur l'analyse
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 2 - Repr. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique