1171455
USP
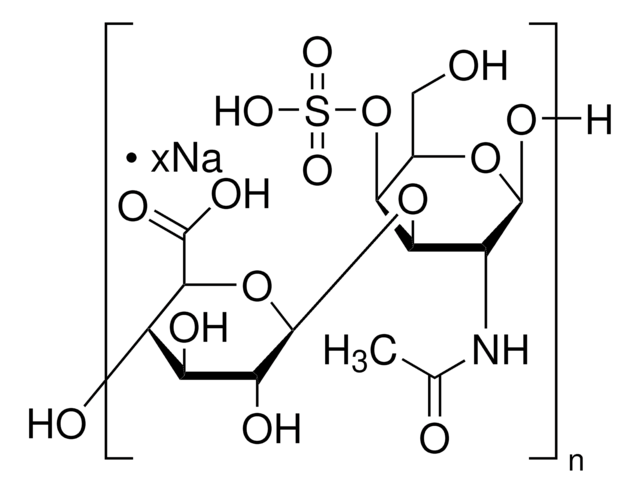
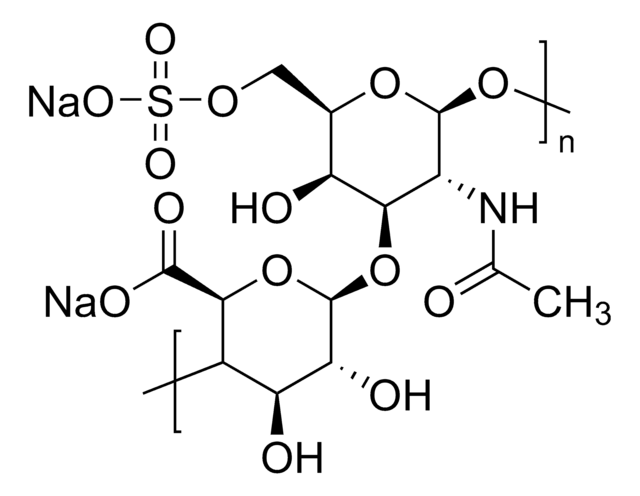
Dermatan sulfate
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Chondroitin sulfate B sodium salt, β-Heparin, Dermatan sulfate sodium salt
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
dermatan sulfate
Forme
solid
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Dermatan sulfate is a sulfated glycosaminoglycan structurally related to heparin, found in animal connective tissues. It plays a role in anticoagulation and cellular signaling. The USP standard is used to characterize dermatan sulfate content and identity in complex glycosaminoglycan mixtures.
The USP biologics carbohydrates category includes a variety of carbohydrate-based substances that are essential in the development and manufacturing of therapeutic products. These carbohydrates play crucial roles in biological processes and are often utilized as excipients, stabilizers, or active ingredients in pharmaceuticals. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of carbohydrate therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Dermatan sulfate USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia
Application
Further information is available in the monograph, Heparin Sodium, USP43-NF38 - 2195 of the USP (United States Pharmacopeia).
Autres remarques
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique