1005706
USP
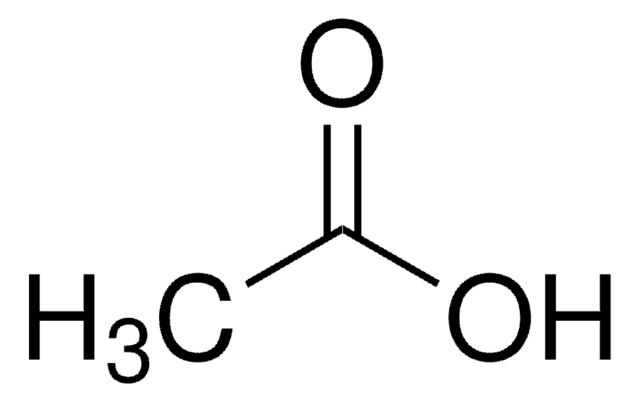
Acide acétique glacial
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Acide acétique, Acide acétique glacial
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Densité de vapeur
2.07 (vs air)
Famille d'API
glacial acetic acid
Forme
liquid
Température d'inflammation spontanée
800 °F
Limite d'explosivité
16 %, 92 °F
4 %, 59 °F
Fabricant/nom de marque
USP
Technique(s)
gas chromatography (GC): suitable
Indice de réfraction
n20/D 1.371 (lit.)
pb
117-118 °C (lit.)
Pf
16.2 °C (lit.)
Densité
1.04 g/mL at 25 °C (lit.)
Application(s)
pharmaceutical (small molecule)
Format
neat
Chaîne SMILES
[F2C(F2C)13F3C]C(O)=O
InChI
1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)
Clé InChI
QTBSBXVTEAMEQO-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Glacial acetic acid is a commonly used solvent and pH modifier in peptide synthesis and purification. It may be present as a residual process reagent. The USP standard is used to quantify acetic acid levels in peptide APIs and assess formulation stability.
The USP biologics peptides category encompasses a diverse range of therapeutic peptides that are essential in managing various medical conditions. These peptides, typically consisting of amino acid sequences of 40 residues or less, are critical for the development of high-quality medicines. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of peptide therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Application
Further information is available in the general chapter, 503- ACETIC ACID IN PEPTIDES, USP43-NF38 - 6741 of the USP (United States Pharmacopeia).
Autres remarques
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
102.2 °F - closed cup
Point d'éclair (°C)
39 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Butyl methyl ether; Acetic acid; 2-Butanone; Ethyl acetate; Tetrahydrofuran; 1-Butanol; Isopropyl acetate; Heptane; Propyl acetate; 3-Methylbutanol; 4-Methyl-2-pentanone; Isobutyl acetate; Butyl acetate; Dimethyl sulfoxide; Anisole; Cumene
Protocoles
Separation of Salicylic acid, meets analytical specification of Ph. Eur., BP, USP, 99.5-100.5% (calc. to the dried substance); Acetylsalicylic acid, purum, ≥99.0% (HPLC)
Separation of Pyruvic acid, United States Pharmacopeia (USP) Reference Standard; Tartaric acid, United States Pharmacopeia (USP) Reference Standard; Citric acid, United States Pharmacopeia (USP) Reference Standard; Malic acid, United States Pharmacopeia (USP) Reference Standard; L-Pyroglutamic acid, ≥99.0% (T); Lactic acid, United States Pharmacopeia (USP) Reference Standard; Acetic acid, ≥99.99% trace metals basis; Succinic acid, United States Pharmacopeia (USP) Reference Standard
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Separation of Acetone; Acetic acid; Propionic acid; Ethyl butyrate; Ethanol; Isoamyl acetate; Isobutyric acid; 3-Methyl-2-butanol; Methyl acetate; 1-Propanol; Acetal, ≥98%, FG; 2-Methyl-1-pentanol; Butyl acetate; Ethyl propionate; 3-Pentanol; 2-Pentanol, 98%; Ethyl isobutyrate; Isobutyl acetate; Acetaldehyde; Furfural; Butyric acid; Methanol; Ethyl acetate
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique