ABN1650
Anti-Beta (β)-Amyloid antibody
rabbit polyclonal
Synonyme(s) :
Aβ-42 oligomer, Abeta-42 oligomer, Oligomeric Aβ-42, Oligomeric Abeta-42
About This Item
Produits recommandés
Nom du produit
Anti-Aβ-42 Antibody, oligomeric (VIA), serum, from rabbit
Source biologique
rabbit
Niveau de qualité
Forme d'anticorps
serum
Type de produit anticorps
primary antibodies
Clone
polyclonal
Espèces réactives
human
Technique(s)
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
western blot: suitable
Numéro d'accès UniProt
Conditions d'expédition
ambient
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... APP(351)
Description générale
Spécificité
Immunogène
Application
Immunohistochemistry Analysis: A 1:150 dilution from a representative lot detected oligomeric Aβ-42 immunoreactivity in Alzheimer′s diseased (AD) human cortex cryosections (Courtesy of Riddhi U Bodani, Kayed lab, Univ of Texas Medical Branch, Galveston, U.S.A.).
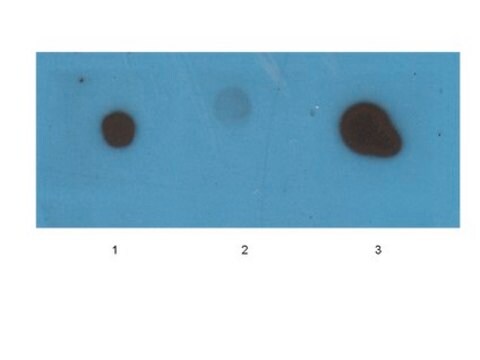
Dot Blot Analysis: A representative lot detected recombinant Aβ-42, but not Aβ-40, oligomers (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Immunofluorescence Analysis: A representative lot immunostained both prefibrillar and fibrillar Aβ-42 oligomers in Alzheimer′s diseased (AD) human brain (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Immunofluorescence Analysis: A representative lot detected oligomeric Aβ-42, but not amyloid plaques in coronal sections of Tg2576AD mice harboring human Swedish mutation APP (APPK670/671L) transgene (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Immunohistochemistry Analysis: A representative lot immunostained intracellular perinuclear Aβ-42 aggregates, but not amyloid plaques in Alzheimer′s diseased (AD) human brain (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Immunohistochemistry Analysis: A representative lot detected oligomeric Aβ-42 immunoreactivity in the hippocampus CA1 region of Tg2576AD mice harboring human Swedish mutation APP (APPK670/671L) transgene (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Western Blotting Analysis: A representative lot detected oligomeric A -42, but not monomeric Aβ-42, monomeric or oligomeric Aβ-40 (Bodani, R.U., et al. (2015). ACS Chem. Neurosci. 6(12):1981-1989).
Note: Both purified antibody (Cat. No. ABN1665) and unpurified antiserum (Cat. No. ABN1650) are suitable for Dot blot, immunofluorescence, immunohistochemistry, and Western blotting applications. However, we recommend using only the purified antibody for neutralization studies.
Neuroscience
Qualité
Dot Blot Analysis: A 1:150 dilution of this antiserum detected recombinant Aβ-42, but not Aβ-40, oligomer.
Description de la cible
Forme physique
Stockage et stabilité
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Autres remarques
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique