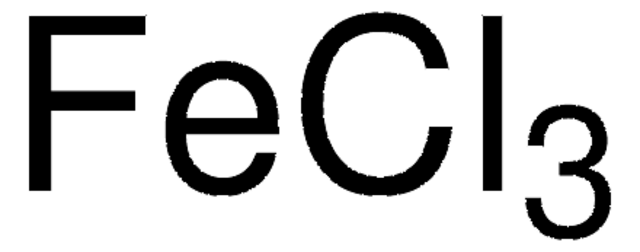940534
Iron(III) chloride hexahydrate

≥99.99% trace metals basis
Synonyme(s) :
Ferric chloride hexahydrate, Iron(III) chloride hexahydrate
About This Item
Produits recommandés
Description
product type - High purity Salts
Niveau de qualité
Essai
≥99.99% trace metals basis
Forme
powder or crystals
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
(≤100ppm trace metals basis)
Pf
37 °C
Solubilité
water: soluble
Traces d'anions
nitrate (NO3-): ≤20 ppm
sulfate (SO42-): ≤20 ppm
Traces de cations
Al: ≤10 ppm
B: ≤10 ppm
Ba: ≤10 ppm
Ca: ≤10 ppm
Co: ≤10 ppm
Cr: ≤10 ppm
Cu: ≤10 ppm
K: ≤10 ppm
Mg: ≤10 ppm
Mn: ≤10 ppm
Na: ≤10 ppm
Ni: ≤10 ppm
Pb: ≤10 ppm
Si: ≤10 ppm
Ti: ≤10 ppm
Zn: ≤10 ppm
Autre catégorie plus écologique
Chaîne SMILES
Cl[Fe](Cl)Cl.O
InChI
InChI=1S/3ClH.Fe.6H2O/h3*1H;;6*1H2/q;;;+3;;;;;;/p-3
Clé InChI
NQXWGWZJXJUMQB-UHFFFAOYSA-K
Catégories apparentées
Description générale
Application
- To synthesize a trimetallic Metal–Organic Frameworks (Fe/Ni/Co/(Mn)-MIL-53) by solvothermal method as a electrocatalyst that exhibits a volcano-type oxygen evolution reaction (OER)activity as a function of compositions.
- To synthesis Fe(III) based MOF such as MIL-53(Fe) by solovothermal method.These MOFs have demonstrated promising photocatalytic properties for both the reduction of Cr(VI) and the oxidation of dyes . Furthermore, they have shown potential in the degradation of organic pollutants when exposed to visible LED light, with persulfate serving as a mediator.
- To synthesize of Magnetite Fe3O4 nanoparticles by wet chemical reduction method.Additionally, monodisperse Fe3O4/C core-shell nanosheets can be synthesized using the carbothermal reduction method for greatly improved microwave absorption.
- To synthesize of Fe3O4@rGO composite electrodes with a core-void-shell structure for Lithium-ion batteries.The resulting electrodes exhibit enhanced electrochemical performance, surpassing that of electrodes based solely on the bare Fe3O4 material. Notable improvements include higher discharge-charge capacity, improved cycling stability, and enhanced rate capability.
Iron(III) chloride hexahydrate serves as a crucial precursor for the synthesis of iron-based composites utilized in wastewater treatment applications. These composites demonstrate excellent potential for effectively treating wastewater and removing contaminants.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique