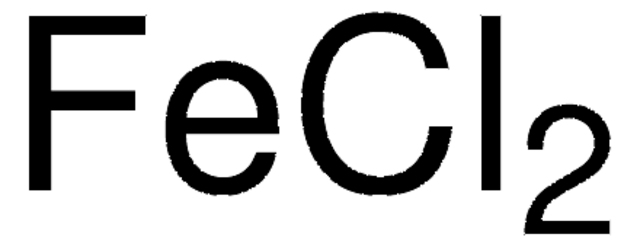939935
Iron(II) chloride anhydrous

≥99.99% trace metals basis
Synonyme(s) :
Iron(II) chloride, Ferrous chloride, Iron dichloride
About This Item
Produits recommandés
Niveau de qualité
Essai
≥99.99% trace metals basis
Forme
powder or crystals
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
≤100 ppm (trace metals analysis)
Pf
677 °C (lit.)
Solubilité
water: soluble
Densité
3.16 g/mL at 25 °C (lit.)
3.16 g/mL at 25 °C
Traces d'anions
chlorate, nitrate (as NO3-): ≤20 ppm
sulfate (SO42-): ≤20 ppm
≤50 ppm (oxygen)
Traces de cations
Al: ≤10 ppm
B: ≤10 ppm
Ba: ≤10 ppm
Ca: ≤10 ppm
Co: ≤10 ppm
Cr: ≤10 ppm
Cu: ≤10 ppm
K: ≤10 ppm
Mg: ≤10 ppm
Mn: ≤10 ppm
Na: ≤10 ppm
Ni: ≤10 ppm
Si: ≤10 ppm
Ti: ≤10 ppm
Zn: ≤10 ppm
Autre catégorie plus écologique
Température de stockage
2-30°C
Chaîne SMILES
Cl[Fe]Cl
InChI
1S/2ClH.Fe/h2*1H;/q;;+2/p-2
Clé InChI
NMCUIPGRVMDVDB-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Caractéristiques et avantages
- Anhydrous
- Low water content
- Low Oxygen content
-Perfect use for molten salt reactors
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique