M4295
Maltotriitol
≥95%
Synonym(s):
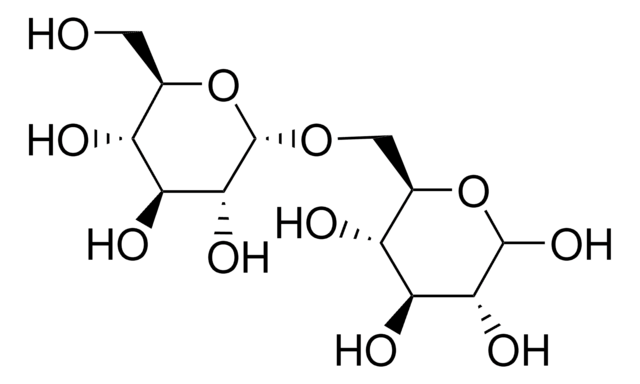
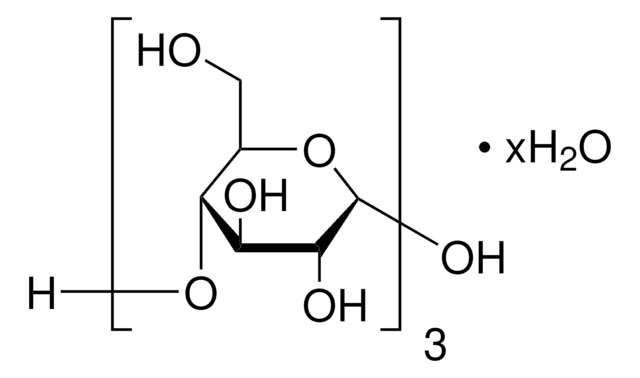
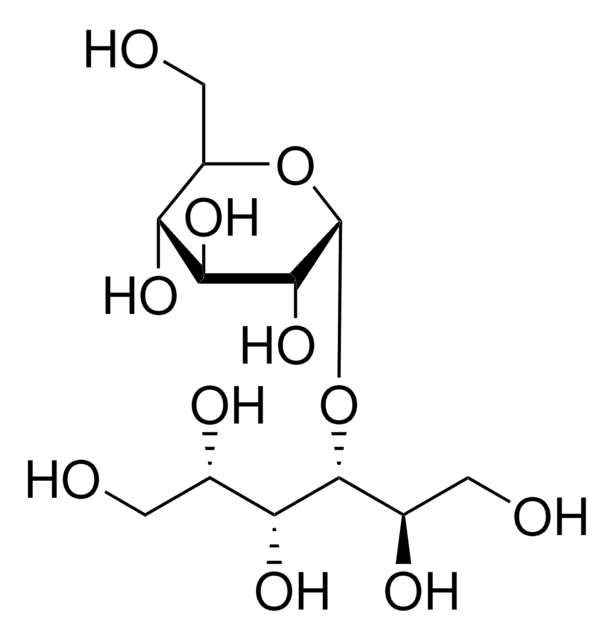
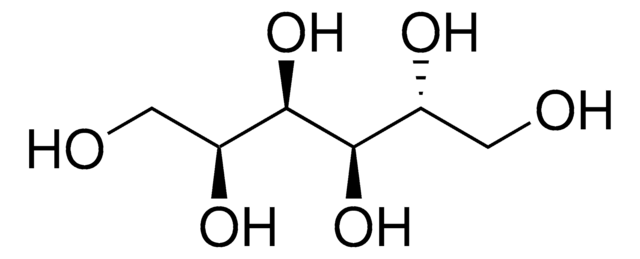
α-D-Glc-(1→4)-α-D-Glc-(1→4)-D-glucitol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C18H34O16
CAS Number:
Molecular Weight:
506.45
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic (organic)
Quality Level
assay
≥95%
form
powder
storage temp.
−20°C
SMILES string
OCC(O)C(O)C(OC1OC(CO)C(OC2OC(CO)C(O)C(O)C2O)C(O)C1O)C(O)CO
InChI
1S/C18H34O16/c19-1-5(23)9(25)15(6(24)2-20)33-18-14(30)12(28)16(8(4-22)32-18)34-17-13(29)11(27)10(26)7(3-21)31-17/h5-30H,1-4H2
InChI key
XJCCHWKNFMUJFE-UHFFFAOYSA-N
General description
Maltotriitol is a sugar.
Application
The presence of maltotriitol (C(18)H(34)O(16)) in maltose syrup is responsible for a change of the crystal morphology in the industrial crystallization process of maltitol (C(12)H(24)O(11)). Maltotriitol can be used as an inhibitor of acid production in human dental plaque.
Other Notes
To gain a comprehensive understanding of our extensive range of Sugar alcohols for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Inhibition of human digestive enzymes by hydrogenated malto-oligosaccharides
Wursch, P. and S. Vedovo
Internat. J. Vit. Nutr. Res., 51, 161-165 (1981)
Maltotriitol inhibition of maltose metabolism in Streptococcus mutans via maltose transport, amylomaltase and phospho-alpha-glucosidase activities.
P Würsch et al.
Caries research, 19(5), 439-449 (1985-01-01)
S Aizawa et al.
Caries research, 43(1), 17-24 (2009-01-13)
This study evaluated acid production from cooked starch by Streptococcus mutans, Streptococcus sobrinus, Streptococcus sanguinis and Streptococcus mitis, and the effects of alpha-amylase inhibitors (maltotriitol and acarbose) and xylitol on acid production. Streptococcal cell suspensions were anaerobically incubated with various
H Kondo et al.
Carbohydrate research, 206(1), 161-166 (1990-09-30)
The effect of the oligosaccharide analog maltotriitol (G3OH) on the action pattern of porcine pancreatic alpha-amylase (PPA) was examined using amylose as a substrate. Fluorescence titration indicated that two molecules of G3OH can bind to one molecule of PPA. The
F Capet et al.
Carbohydrate research, 339(6), 1225-1231 (2004-04-06)
In the industrial crystallisation process of maltitol (C(12)H(24)O(11)), the presence of maltotriitol (C(18)H(34)O(16)) in the maltose syrup is responsible for a change of the crystal morphology. Two different crystal forms of maltitol were obtained: a prismatic one and a 'bipyramidal'
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service