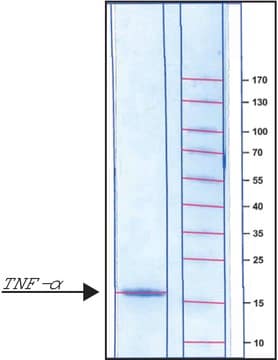
H8916
Tumor Necrosis Factor-α human
≥95% (SDS-PAGE), recombinant, expressed in HEK 293 cells, lyophilized powder, suitable for cell culture
Synonym(s):
TNF-α
About This Item
Recommended Products
Product Name
Tumor Necrosis Factor-α human, Xeno-free, recombinant, expressed in HEK 293 cells, suitable for cell culture
biological source
human
Quality Level
recombinant
expressed in HEK 293 cells
assay
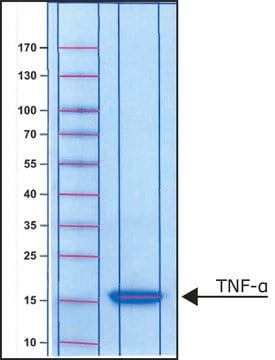
≥95% (SDS-PAGE)
form
lyophilized powder
potency
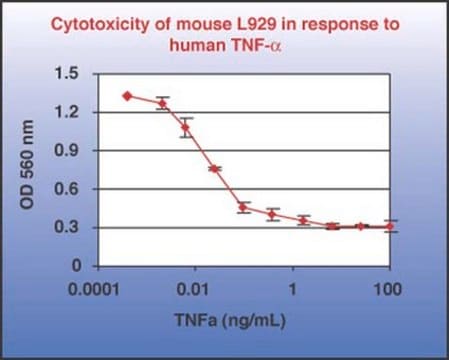
≤1.0 ng/mL ED50
quality
endotoxin tested
mol wt
17 kDa (glycosylated)
~17.4 kDa
packaging
pkg of 10 μg
storage condition
avoid repeated freeze/thaw cycles
technique(s)
cell culture | mammalian: suitable
impurities
≤1.00 EU/μg (endotoxin)
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... TNF(7124)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In induction of netting neutrophils by anti-neutrophil cytoplasmic antibody and to study its effect on platelet activation and formation of monomeric C-reactive protein.
- To study the effect of TNF-α on miR-221 and fractalkine expression.
- To induce inflammatory cell responses.
- In NF-κB luciferase reporter assay.
- as a permeability inducing agent for endothelial cell monolayer permeability assay
- as a reactive oxygen species inducer in primary rat cardiac microvascular endothelial cells (RCMVECs)
- in the activation of nuclear factor kappa B (NF-κB) in human embryonic kidney cells (HEK293), neuroblastoma SH-SY5Y cells and HeLa cells
- in the stimulation of the human keratinocyte cell line(HaCaT) and human coronary artery endothelial cells (HCAECs)
Biochem/physiol Actions
Preparation Note
Analysis Note
comparable product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2 Replication of Classical Swine Fever Virus
Articles
Lipid Induced Insulin Resistance
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service