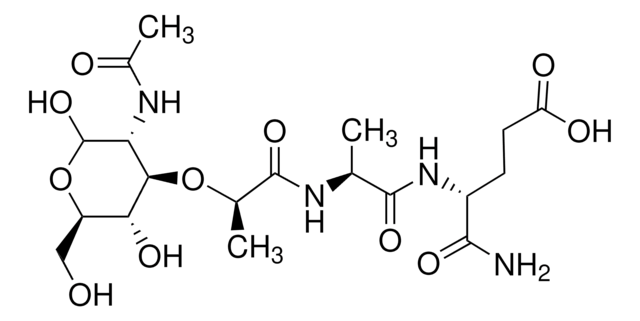
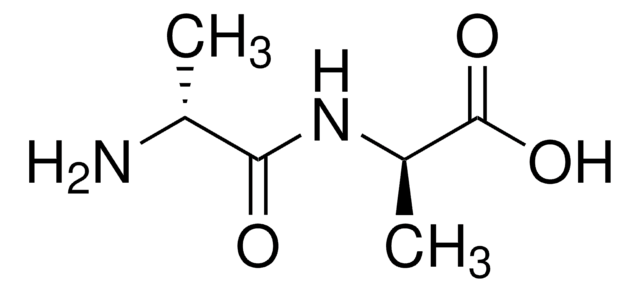
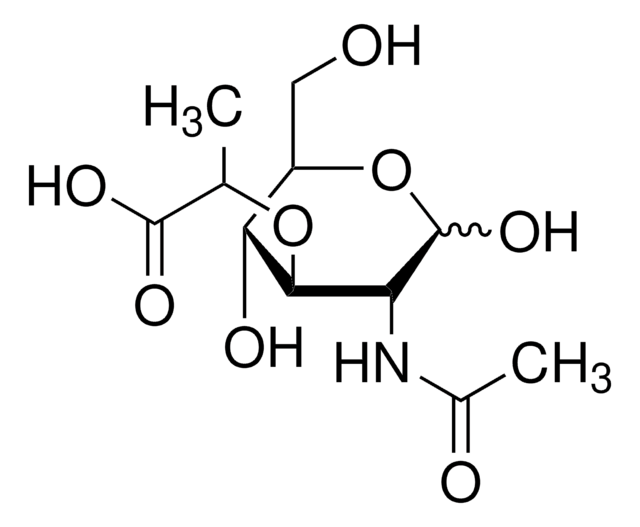
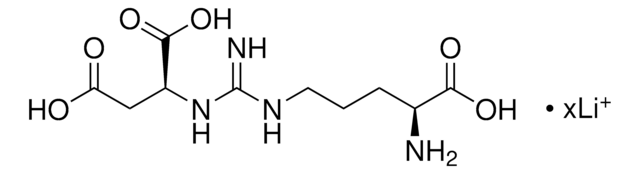
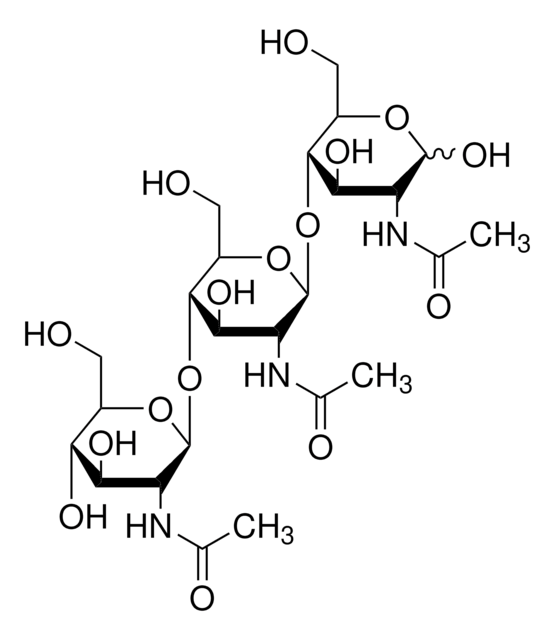
A0910
Ala-D-γ-Glu-Lys-D-Ala-D-Ala
≥97% (HPLC)
Synonym(s):
Peptidoglycan pentapeptide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H36N6O8
CAS Number:
Molecular Weight:
488.54
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
assay
≥97% (HPLC)
form
powder
color
white
storage temp.
−20°C
SMILES string
CC(N)C(=O)NC(CCC(=O)NC(CCCCN)C(=O)NC(C)C(=O)NC(C)C(O)=O)C(O)=O
InChI
1S/C20H36N6O8/c1-10(22)16(28)26-14(20(33)34)7-8-15(27)25-13(6-4-5-9-21)18(30)23-11(2)17(29)24-12(3)19(31)32/h10-14H,4-9,21-22H2,1-3H3,(H,23,30)(H,24,29)(H,25,27)(H,26,28)(H,31,32)(H,33,34)
InChI key
MCIBYIIODNXVSL-UHFFFAOYSA-N
Related Categories
Amino Acid Sequence
Ala-Glu-Lys-Ala-Ala
Application
Ala-D-γ-Glu-Lys-D-Ala-D-Ala is the pentapeptide tail of the peptidoglycan precursor UDPMurNAc-l-Ala-γ-d-Glu-l-Lys(Gly)(5)-d-Ala-d-Ala. Ala-D-γ-Glu-Lys-D-Ala-D-Ala is used to study the role of this peptide stem in peptidoglycan biosynthesis, degradation and function.
Biochem/physiol Actions
L-Ala-D-Glu-gamma-L-Lys-D-Ala-D-Ala, peptidoglycan pentapeptide, is the antigenic peptide determinant of peptidoglycan useful in studies of mechanisms of action of glycopeptide antibiotics.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Heymer et al.
Journal of immunology (Baltimore, Md. : 1950), 117(1), 23-26 (1976-07-01)
A radioimmunoassay has been developed for the measurement of antibodies to peptidoglycan in human sera including patients with rheumatic fever and juvenile rheumatoid arthritis. The assay is based on the percentage of binding of the hapten 125I-L-Ala-gamma-d-Glu-L-Lys-D-Ala-D-Ala, the major peptide
B Heymer et al.
Zeitschrift fur Immunitatsforschung, experimentelle und klinische Immunologie, 149(2-4), 168-178 (1975-07-01)
Staphylococcus epidermidis peptidoglycans solubilized by sonication or lysozyme digestion, and synthetic peptidoglycan analogs such as HSA-carboxymethyl-Gly-L-Ala-L-Ala-D-Ala-D-Ala (HSA-pentapeptide) or L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala (pentapeptide) have been labeled with 125I and tested for their applicability in the radioactive antigen binding assay. Use of radioiodinated Staph.
Ahmed Bouhss et al.
The Journal of biological chemistry, 277(48), 45935-45941 (2002-09-27)
The enzymatic synthesis of the complete l-alanyl(1)-l-alanine(2) side chain of the peptidoglycan precursors of Enterococcus faecalis was obtained in vitro using purified enzymes. The pathway involved alanyl-tRNA synthetase and two ligases, BppA1 and BppA2, that specifically transfer alanine from Ala-tRNA
Lorena Abadía Patiño et al.
Antimicrobial agents and chemotherapy, 49(4), 1419-1425 (2005-03-29)
Enterococcus faecalis BM4405-1, a susceptible derivative of the VanE-type vancomycin-resistant E. faecalis strain BM4405, was obtained after growth in the presence of novobiocin, an inhibitor of the GyrB subunit of DNA gyrase. In contrast to findings for BM4405, UDP-MurNAc-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala (pentapeptide[D-Ala])
Sridhar Peddi et al.
Biochemistry, 48(24), 5731-5737 (2009-05-06)
Penicillin-binding proteins (PBPs) are bacterial enzymes involved in the final stages of cell wall biosynthesis and are the lethal targets of beta-lactam antibiotics. Despite their importance, their roles in cell wall biosynthesis remain enigmatic. A series of eight substrates, based
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service