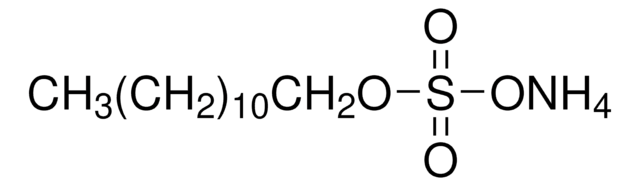09887
Ammonium lauryl sulfate solution
~30% in H2O (T)
Synonym(s):
Ammonium dodecyl sulfate, Dodecyl sulfate ammonium salt
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
CH3(CH2)11OSO3NH4
CAS Number:
Molecular Weight:
283.43
Beilstein/REAXYS Number:
5188379
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
description
anionic
Quality Level
form
liquid
mol wt
283.43 g/mol
concentration
~30% in H2O (T)
color
Colorless to Very Light Yellow and Colorless to Very Light Green-Yellow
refractive index
n20/D 1.37
pH
6.8
solubility
water: soluble at 20 °C
density
1.02 g/mL at 20 °C
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium lauryl sulfate is an anionic surfactant consisting of a nonpolar hydrocarbon chain and a polar sulfate end group. It is used as a foaming agent, and as a detergent in many industrial applications.
Application
Ammonium lauryl sulfate can be used as:
- A catalyst in the synthesis of 1H-benzo[d]imidazole, quinoxaline, and 2,3-dihydro-1H-benzo[b][1,4]diazepine derivatives by reacting with benzene-1,2 diamine and aromatic aldehydes/1,2-diketones/ketones respectively.
- A corrosion inhibitor for carbon steel and copper in acidic solution.
- A surfactant in the fabrication of porous ceramics by gel casting.
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bryan F Shaw et al.
Journal of the American Chemical Society, 133(44), 17681-17695 (2011-09-24)
A previous study, using capillary electrophoresis (CE) [J. Am. Chem. Soc. 2008, 130, 17384-17393], reported that six discrete complexes of ubiquitin (UBI) and sodium dodecyl sulfate (SDS) form at different concentrations of SDS along the pathway to unfolding of UBI
Shobha N Bhattachar et al.
International journal of pharmaceutics, 412(1-2), 95-98 (2011-04-30)
This work reports on the solubility of two weakly basic model compounds in media containing sodium lauryl sulfate (SLS). Results clearly show that the presence of SLS in the media (e.g. simulated gastric fluid or dissolution media) can result in
Robert Renthal et al.
Biophysical chemistry, 159(2-3), 321-327 (2011-09-20)
To probe structural changes that occur when a membrane protein is transferred from lipid bilayers to SDS micelles, a fragment of bacteriorhodopsin containing transmembrane helical segments A and B was studied by fluorescence spectroscopy, molecular dynamics (MD) simulation, and stopped
Minmin Teng et al.
Journal of colloid and interface science, 338(2), 537-541 (2009-07-21)
A Mg2+-induced vesicle phase was prepared from a mixture of tetradecyldimethylamine oxide (C14DMAO) and magnesium dodecyl sulfate [Mg(DS)2] in aqueous solution. Study of the phase behavior shows that at the appropriate mixing ratios, Mg2+-ligand coordination between C14DMAO and Mg(DS)2 results
Lilia Fransisca et al.
Journal of food science, 77(2), M121-M126 (2012-02-09)
It has been reported that washing seeds with a 20000 ppm Ca(OCl)(2) solution as recommended by the U.S. Food and Drug Administration is unable to eliminate E. coli cells attached to seed surfaces, and the bacterial cells that have survived
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service