05-808
Anti-dimethyl-Histone H3 (Arg2) Antibody, rabbit monoclonal
Upstate®, from rabbit
Synonym(s):
H3R2me2, Histone H3 (di methyl R2)
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
rabbit
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
monoclonal
species reactivity
human
manufacturer/tradename
Upstate®
technique(s)
dot blot: suitable
inhibition assay: suitable (peptide)
multiplexing: suitable
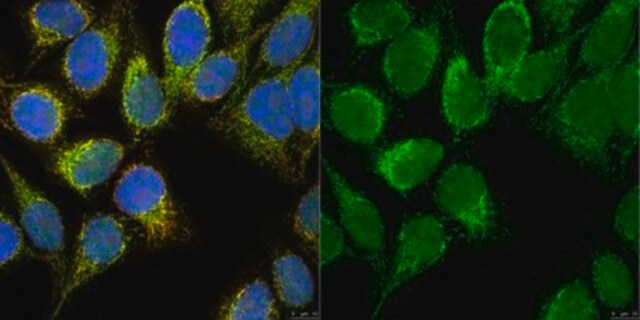
western blot: suitable
isotype
IgG
NCBI accession no.
General description
Histone H3 is one of the five main histone proteins involved in the structure of chromatin in eukaryotic cells. Featuring a main globular domain and a long N-terminal tail, H3 is involved with the structure of the nucleosomes of the ′beads on a string′ structure.
The N-terminal tail of histone H3 protrudes from the globular nucleosome core and can undergo several different types of epigenetic modifications that influence cellular processes. These modifications include the covalent attachment of methyl or acetyl groups to lysine and arginine amino acids and the phosphorylation of serine or threonine.
The N-terminal tail of histone H3 protrudes from the globular nucleosome core and can undergo several different types of epigenetic modifications that influence cellular processes. These modifications include the covalent attachment of methyl or acetyl groups to lysine and arginine amino acids and the phosphorylation of serine or threonine.
Immunogen
peptide containing the sequence AdimRTKQ, corresponding to dimethyl-arginine 2 of human histone H3.
Application
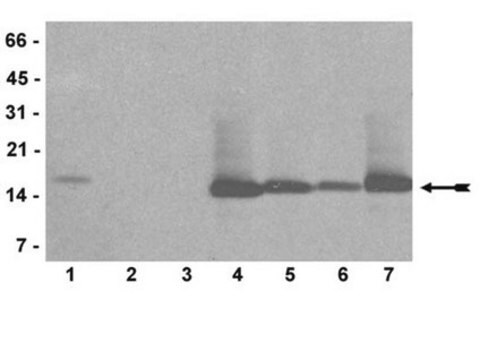
Use Anti-dimethyl-Histone H3 (Arg2) Antibody (Rabbit Monoclonal Antibody) validated in DB, WB, PIA, Mplex to detect dimethyl-Histone H3 (Arg2) also known as H3R2me2, Histone H3 (di methyl R2).
Quality
routinely evaluated in acid-extracted proteins from HeLa cells
Target description
17kDa
Physical form
Format: Purified
Analysis Note
Control
Acid extracts from HeLa cells
Acid extracts from HeLa cells
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zichuan Liu et al.
Developmental biology, 368(2), 304-311 (2012-06-05)
Several research groups have suggested that the embryonic-abembryonic (Em-Ab) axis in the mouse can be predicted by the first cleavage plane of the early embryo. Currently, it is not known whether this early patterning occurs in cloned embryos produced by
Lessly P Sepulveda-Rincon et al.
Biology of reproduction, 95(6), 123-123 (2016-10-21)
The first lineage specification during mammalian embryo development can be visually distinguished at the blastocyst stage. Two cell lineages are observed on the embryonic-abembryonic axis of the blastocyst: the inner cell mass and the trophectoderm. The timing and mechanisms driving
Alessandra Di Lorenzo et al.
Nucleic acids research, 42(13), 8297-8309 (2014-06-19)
Protein arginine methyltransferase 6 (PRMT6) is a nuclear enzyme that modifies histone tails. To help elucidate the biological function of PRMT6 in vivo, we generated transgenic mice that ubiquitously express PRMT6 fused to the hormone-binding portion of the estrogen receptor
Ayaz Ahmad et al.
PloS one, 6(8), e22664-e22664 (2011-08-20)
Post-translational methylation of arginine residues profoundly affects the structure and functions of protein and, hence, implicated in a myriad of essential cellular processes such as signal transduction, mRNA splicing and transcriptional regulation. Protein arginine methyltransferases (PRMTs), the enzymes catalyzing arginine
Analysis of histones in Xenopus laevis. I. A distinct index of enriched variants and modifications exists in each cell type and is remodeled during developmental transitions.
Shechter, D; Nicklay, JJ; Chitta, RK; Shabanowitz, J; Hunt, DF; Allis, CD
The Journal of Biological Chemistry null
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service