All Photos(2)
About This Item
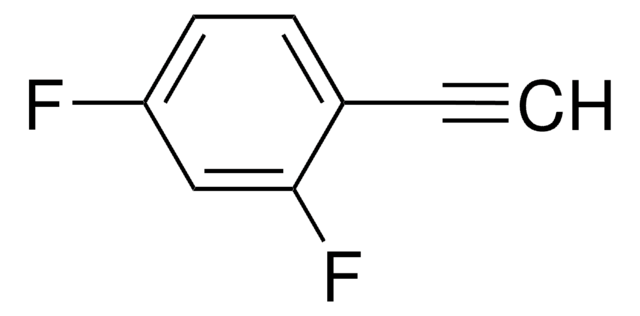
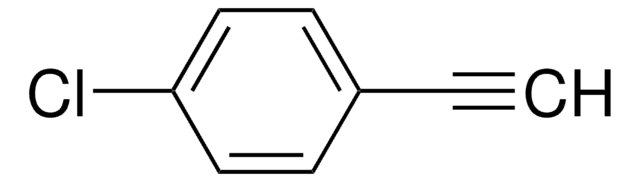
Linear Formula:
FC6H4C≡CH
CAS Number:
Molecular Weight:
120.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.526 (lit.)
bp
150 °C (lit.)
density
1.06 g/mL at 25 °C (lit.)
functional group
fluoro
storage temp.
2-8°C
SMILES string
Fc1ccccc1C#C
InChI
1S/C8H5F/c1-2-7-5-3-4-6-8(7)9/h1,3-6H
InChI key
YFPQIXUNBPQKQR-UHFFFAOYSA-N
General description
1-Ethynyl-2-fluorobenzene, a fluorinated benzene derivative, is a terminal alkyne.
Application
1-Ethynyl-2-fluorobenzene has been employed in the cross-coupling of phenylacetylenes. It may be used in the preparation of 3-(2-deoxy-β-D-ribofuranosyl)-6-(2-fluorophenyl)-2,3-dihydrofuro-[2,3-d]pyrimidin-2-one and 4-(2-fluorophenylethynyl)-3-methyl-5-phenylisoxazole.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
98.6 °F - closed cup
flash_point_c
37 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Biological Evaluation of Novel 1, 2, 3-Triazolonucleotides.
Glowacka IE, et al.
Arch. Pharm. (Weinheim), 346(4), 278-291 (2013)
Synthesis of 3, 4, 5-Trisubstituted Isoxazoles through Gold-Catalyzed Cascade Cyclization- Oxidative Alkynylation and Cyclization-Fluorination of 2-Alkynone O-Methyloximes.
Song D-H and Ryu J-S.
Bull. Korean Chem. Soc., 35(19), 2635-2644 (2014)
Synthesis of Novel 1-Hydroxy-2-(1, 2, 3-triazol-1-yl) ethylphosphonates and 2-Hydroxy-3-(1, 2, 3-triazol-1-yl) propylphosphonates.
Glowacka IE, et al.
Phosphorus, Sulfur, and Silicon and the Related Elements, 186(3), 431-449 (2011)
Tetrahedron Letters, 35, 3689-3689 (1994)
Halophenyl furanopyrimidines as potent and selective anti-VSV agents.
McGuigan C, et al.
Antiviral Chem. Chemother., 14(3), 165-165 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service