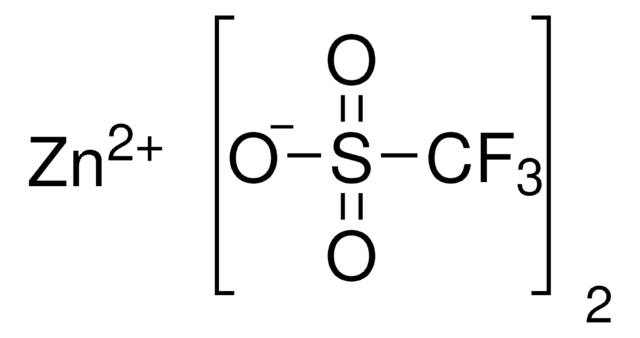190357
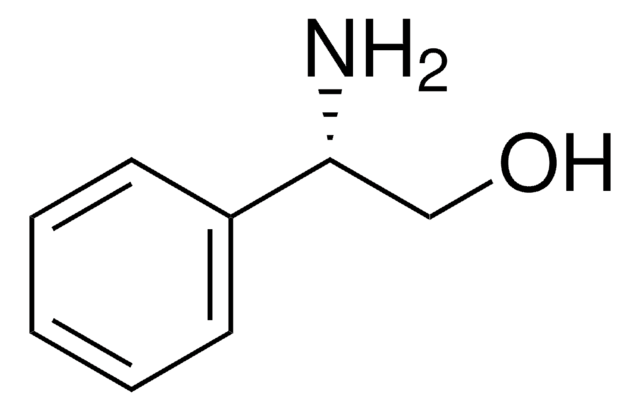
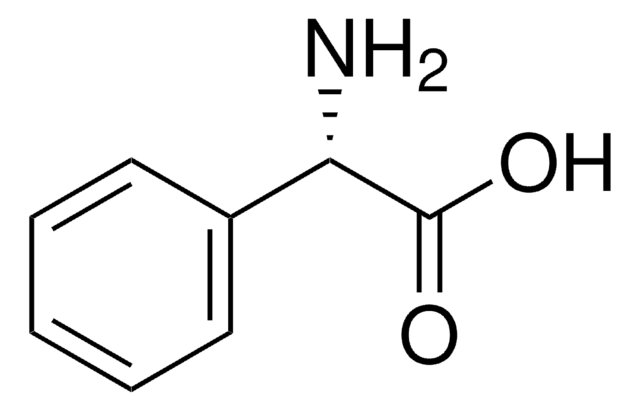
(R)-(−)-2-Phenylglycinol
98%, for peptide synthesis
Synonym(s):
(R)-2-Amino-2-phenylethanol, D-(−)-α-Phenylglycinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH(NH2)CH2OH
CAS Number:
Molecular Weight:
137.18
Beilstein/REAXYS Number:
2935848
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
(R)-(−)-2-Phenylglycinol, 98%
Quality Level
assay
98%
optical activity
[α]24/D −31.7°, c = 0.76 in 1 M HCl
optical purity
ee: 99% (GLC)
reaction suitability
reaction type: solution phase peptide synthesis
mp
75-77 °C (lit.)
application(s)
peptide synthesis
SMILES string
N[C@@H](CO)c1ccccc1
InChI
1S/C8H11NO/c9-8(6-10)7-4-2-1-3-5-7/h1-5,8,10H,6,9H2/t8-/m0/s1
InChI key
IJXJGQCXFSSHNL-QMMMGPOBSA-N
Related Categories
Application
Amino alcohol used to prepare a chiral imine or oxazolidine from ethyl trifluoropyruvate. These intermediates were then employed in a synthesis of both enantiomers of α-trifluoromethylproline.
Chiral β−amino alcohol used as a synthetic building block.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 45, 5287-5287 (2004)
Amedjkouh, M.; Westerlund, K.
Tetrahedron Letters, 45, 5175-5175 (2004)
Grégory Chaume et al.
Organic letters, 8(26), 6123-6126 (2006-12-15)
[Structure: see text] A concise synthesis of both enantiomers of alpha-Tfm-proline and (S)-alpha-Tfm-prolinol from ethyl trifluoropyruvate is reported. The key step is a diastereoselective allylation reaction of ethyl trifluoropyruvate and (R)-phenylglycinol-based oxazolidines or imine. The lactone obtained by cyclization of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service