All Photos(2)
About This Item
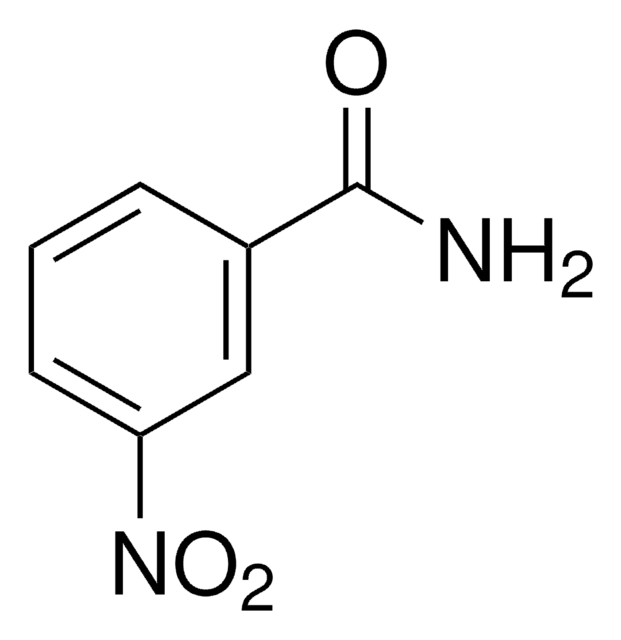
Linear Formula:
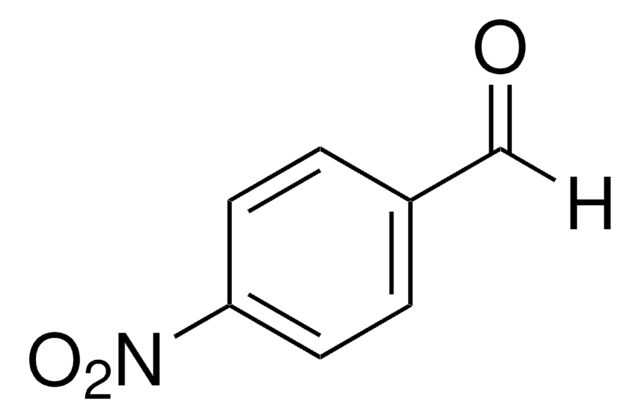
O2NC6H4CONH2
CAS Number:
Molecular Weight:
166.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
199-201 °C (lit.)
functional group
amide
nitro
SMILES string
NC(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H6N2O3/c8-7(10)5-1-3-6(4-2-5)9(11)12/h1-4H,(H2,8,10)
InChI key
ZESWUEBPRPGMTP-UHFFFAOYSA-N
General description
Negative quasimolecular ions of 4-nitrobenzamide has been investigated by electrospray ionization mass spectrometry.
Application
4-Nitrobenzamide was used in the preparation of 4-nitrobenziminosulfurane.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of nitriles from primary amides under Swern oxidation conditions.
Nakajima N and Ubukata M.
Tetrahedron Letters, 38(12), 2099-2102 (1997)
F C Chiu et al.
Journal of the American Society for Mass Spectrometry, 11(12), 1061-1064 (2000-01-11)
Negative quasimolecular ions of aromatic carboxylic acid amides have been observed unexpectedly under electrospray ionization conditions. Hypothetically, deprotonation of either carboxamide or carboximidic acid tautomers can produce anions with equivalent resonance structures, the stability of which is affected by conjugated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service