160954
Methyl 4-nitrobenzenesulfonate
99%
Synonym(s):
Methyl nosylate, Methyl p-nitrobenzenesulfonate, Methyl p-nitrotosylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
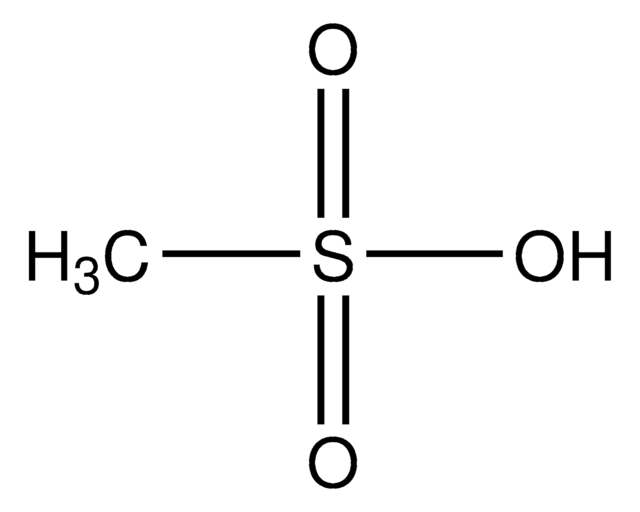
O2NC6H4SO3CH3
CAS Number:
Molecular Weight:
217.20
Beilstein/REAXYS Number:
2277327
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
mp
89-92 °C (lit.)
solubility
acetone: soluble 5%, clear, faintly yellow to greenish-yellow
functional group
nitro
sulfonic acid
SMILES string
COS(=O)(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO5S/c1-13-14(11,12)7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChI key
RMNJNEUWTBBZPT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Reaction between methyl 4-nitrobenzenesulfonate and bromide ions has been studied in mixed single-chain-gemini micellar solutions. Kinetics of SN2 reactions of methyl 4-nitrobenzenesulfonate with ammonia, primary amines, secondary amines, tertiary amines and anionic nucleophiles has been studied.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Ishii et al.
Protein science : a publication of the Protein Society, 7(8), 1802-1810 (1999-03-19)
Aromatic L-amino acid decarboxylase (AADC) catalytic mechanism has been proposed to proceed through two consecutive intermediates (i.e., Michaelis complex and the external aldimine). Limited proteolysis of AADC that preferentially digested at the C-terminal side of Arg334 was slightly retarded in
O Paquatte et al.
Photochemistry and photobiology, 50(6), 817-825 (1989-12-01)
Vibrio harveyi luciferase, an alpha beta dimer, was effectively inactivated by treatment with the methylation agent methyl p-nitrobenzene sulfonate. However, inactivation of luciferase in the presence of excess amounts of this reagent did not follow pseudo-first-order kinetics. After taking the
T Kohzuma et al.
Journal of biochemistry, 106(6), 1054-1058 (1989-12-01)
When Trimeresurus flavoviridis phospholipase A2 was reacted with methyl p-nitrobenzenesulfonate, its activity decreased following first-order kinetics. The pH dependence of the rate constants of inactivation showed that His-48 with an apparent pKa of 6.5 controls the reaction. In the pH
Cysteine modification of metallothionein.
P E Hunziker
Methods in enzymology, 205, 399-400 (1991-01-01)
J P Marcus et al.
Archives of biochemistry and biophysics, 316(1), 413-420 (1995-01-10)
Incubation of L-threonine dehydrogenase from Escherichia coli with methyl p-nitrobenzenesulfonate results in a time- and concentration-dependent loss of enzymatic activity. As the concentration of the methylating agent is increased, the rate of inactivation reaches a limiting value of 0.01 min-1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)


