All Photos(1)
About This Item
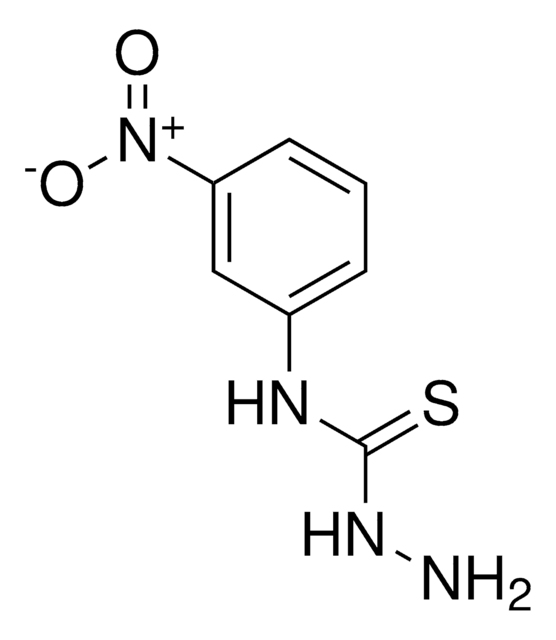
Linear Formula:
C6H5NHCSNHNH2
CAS Number:
Molecular Weight:
167.23
Beilstein/REAXYS Number:
608285
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
mp
138-140 °C (lit.)
functional group
amine
hydrazine
thiourea
SMILES string
NNC(=S)Nc1ccccc1
InChI
1S/C7H9N3S/c8-10-7(11)9-6-4-2-1-3-5-6/h1-5H,8H2,(H2,9,10,11)
InChI key
KKIGUVBJOHCXSP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Phenylthiosemicarbazide was used in the synthesis of amberlite XAD resins. It was also used in the synthesis of a series of thiosemicarbazones.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Acute Tox. 2 Oral
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Justin W Hicks et al.
Chemistry & biodiversity, 5(11), 2415-2422 (2008-11-28)
Addition of thiosemicarbazide, 4-allylthiosemicarbazide, and 4-phenylthiosemicarbazide to (formylphenyl)boronic acids affords a series of thiosemicarbazones containing boronic acids. Addition of 2-formylphenylboronic acid to the thiosemicarbazides gave the corresponding cyclic 2,3,1-benzodiazaborines. All new compounds have been investigated for potential antifungal activity.
Derya Kara et al.
Journal of hazardous materials, 165(1-3), 1165-1169 (2008-12-17)
A matrix separation and analyte preconcentration system using Amberlite XAD copolymer resins functionalized by Schiff base reactions coupled with atomic spectrometry has been developed. Three different functionalized Amberlite XAD resins were synthesized using 4-phenylthiosemicarbazide, 2,3-dihydroxybenzaldehyde and 2-thiophenecarboxaldehyde as reagents. These
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service