F8150
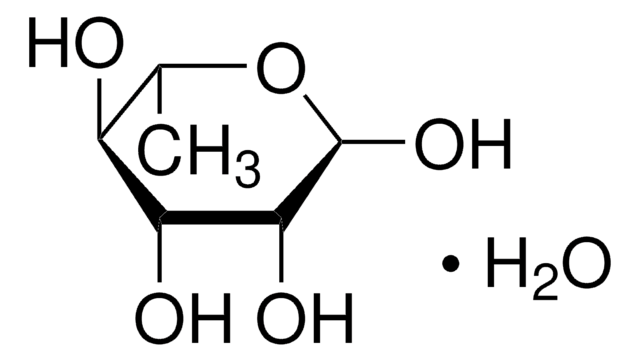
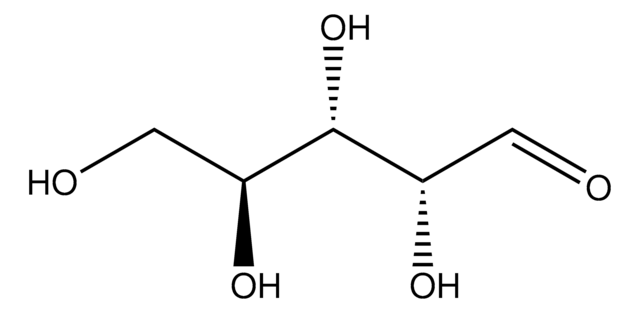
D-(+)-Fucose
≥97% (GC)
Synonym(s):
6-Deoxy-D-galactose, Rhodeose
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C6H12O5
CAS Number:
Molecular Weight:
164.16
Beilstein:
1723320
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥97% (GC)
form
powder
optical activity
[α]20/D 74 to 76 °, c = 4% (w/v) in water
technique(s)
gas chromatography (GC): suitable
color
white
mp
144-145 °C (lit.)
solubility
H2O: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
storage temp.
room temp
Looking for similar products? Visit Product Comparison Guide
Application
D-Fucose (6-deoxy-D-galactose, rhodeose) may be used to study enzymes involved in its mutarotation to form L-fucose. It may be used as a reference compound in the analysis of carbohydrate metabolites. D-Fucose may be used to study biological processes such as adhesion.
Biochem/physiol Actions
D-Fucose is a nonmetabolizable analog of L-Arabinose.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhenzheng Hu et al.
Structure (London, England : 1993), 26(1), 60-71 (2017-12-12)
Mannose receptor (MR, CD206) is an endocytic receptor on microphages and dendritic cells. It recognizes multiple ligands and plays important roles in regulating immune responses and maintaining glycoprotein homeostasis. However, the structure and functional mechanism of MR remain unclear. Here we
P Wlodarczyk et al.
Journal of physics. Condensed matter : an Institute of Physics journal, 25(37), 375101-375101 (2013-08-15)
The sugar specific mutarotation reaction in biologically important L-fucose and its enantiomer in the pure, anhydrous, supercooled liquid state has been studied. Kinetics measurements in the temperature range 313-328 K at ambient pressure have been performed by means of dielectric spectroscopy
Nancy G Isern et al.
Biotechnology for biofuels, 6(1), 47-47 (2013-04-05)
Caldicellulosiruptor saccharolyticus is a thermophilic, Gram-positive, non-spore forming, strictly anaerobic bacterium of interest in potential industrial applications, including the production of biofuels such as hydrogen or ethanol from lignocellulosic biomass through fermentation. High-resolution, solution-state nuclear magnetic resonance (NMR) spectroscopy is
Sarah Meinhardt et al.
Nucleic acids research, 40(21), 11139-11154 (2012-09-12)
LacI/GalR transcription regulators have extensive, non-conserved interfaces between their regulatory domains and the 18 amino acids that serve as 'linkers' to their DNA-binding domains. These non-conserved interfaces might contribute to functional differences between paralogs. Previously, two chimeras created by domain
Xiaonan Zhou et al.
PloS one, 13(10), e0204152-e0204152 (2018-10-09)
Lonicera japonica is a typical Chinese herbal medicine. We previously reported a method to isolate polysaccharides from Lonicera japonica (LJP). In this study, we first performed a qualitative analysis of LJP using the Fourier Transform Infrared Spectrometer (FT-IR) and explored
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service