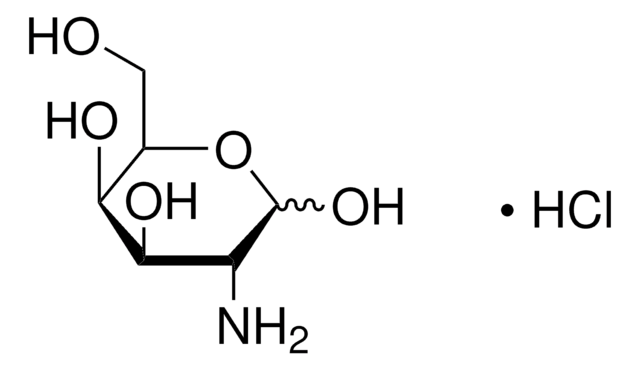
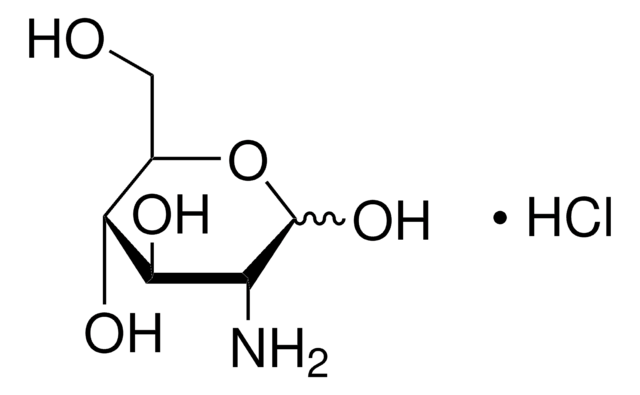
A2267
1-Amino-1-deoxy-β-D-galactose
Synonym(s):
β-D-Galactosylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13NO5
CAS Number:
Molecular Weight:
179.17
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98% (TLC)
Quality Level
form
powder
optical activity
[α]/D 60.00 to 64.00°, c = 18.00-22.00 mg/mL in water
technique(s)
thin layer chromatography (TLC): suitable
storage temp.
2-8°C
SMILES string
NC1OC(CO)C(O)C(O)C1O
InChI
1S/C6H13NO5/c7-6-5(11)4(10)3(9)2(1-8)12-6/h2-6,8-11H,1,7H2
InChI key
WCWOEQFAYSXBRK-UHFFFAOYSA-N
Application
β-D-Galactosylamine, a galactose analogue, is used as a competitive inhibitor to help isolate, purify, identify, differentiate and characterize β-D-galactosidase(s) and galactose oxidase(s).
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C F Mazitsos et al.
Journal of chromatography. A, 954(1-2), 137-150 (2002-06-13)
Two anthraquinone galactosyl-biomimetic dye-ligands comprising, as terminal biomimetic moiety, galactose analogues (1-amino-1-deoxy-beta-D-galactose and D(+)-galactosamine) were designed for the enzyme galactose oxidase (GAO), using molecular modelling, synthesized and characterized. The biomimetic ligands were immobilized on agarose beads and the affinity adsorbents
N Monji et al.
Research communications in chemical pathology and pharmacology, 26(1), 187-196 (1979-10-01)
We have developed a new method for separation of antibody bound and unbound enzyme conjugates. The technique as applied to the assay of choriomammotropin involves the use of beta-D-galactosylamine bound to agarose to separate the unbound choriomammotropin-beta-galactosidase conjugates for antibody
R A DiCioccio et al.
Carbohydrate research, 127(1), 109-120 (1984-04-02)
A beta-D-galactosidase from bovine liver was purified to apparent homogeneity. The major purification step was affinity chromatography on a column of D-galactose attached to a Sepharose support activated with divinyl sulfone. Affinity media prepared by binding ligands to Sepharose activated
Bo-Yu Li et al.
Carbohydrate research, 345(12), 1708-1712 (2010-06-30)
The highly diastereoselective InCl(3)-catalyzed aza-Friedel-Crafts reaction of substituted indoles with aldimines generated from Kunz's amine was studied. The reaction afforded the desired product in good to high yields with up to >19:1 diastereoselective ratios. The O-pivaloylated beta-D-galactosyl moiety could not
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service