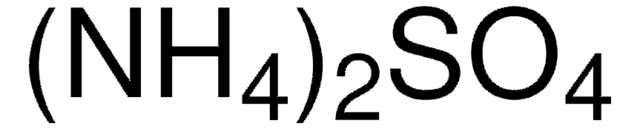09978
Ammonium sulfate
BioUltra, ≥99.0% (T)
Synonym(s):
Mascagnite
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
(NH4)2SO4
CAS Number:
Molecular Weight:
132.14
EC Number:
MDL number:
UNSPSC Code:
12352314
eCl@ss:
38030408
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product line
BioUltra
Quality Level
Assay
≥99.0% (T)
form
crystals
impurities
insoluble matter, passes filter test
ign. residue
≤0.01% (as SO4)
loss
≤0.1% loss on drying, 110 °C
pH
5.0-6.0 (25 °C, 1 M in H2O)
mp
>280 °C (dec.) (lit.)
solubility
H2O: 1 M at 20 °C, clear, colorless
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium sulfate is naturally present as a rare mineral mascagnite in volcanic fumaroles and due to coal fires in some dumps.
Application
Ammonium sulfate has been used:
- to prepare mobile phase A for hydrophobic interaction chromatography (HIC)
- as reference material for the thermal decomposition of NH4NO3 (S1–S6) thermal decomposition to N2O (S1-N2O–S6-N2O)
- to prepare the stock solution for M63 medium
- as a reagent in molecular biology and chromatography
- in the precipitation and fractionation of proteins
- in the purification of antibodies
- for the crystallographic analysis of nucleic acids and proteins
- in high-performance liquid chromatography (HPLC) analysis of proteins, such as in hydrophobic interaction chromatography
- for Sepharose chromatography of tRNAs using reverse salt gradients
- in long PCR buffer, in PCR lysis solution, and second-strand cDNA synthesis
- HPLC analysis of phospholipids
Biochem/physiol Actions
Ammonium sulfate is an inorganic salt, which is mostly used as a soil fertilizer. It is also used as a flame retardant since it elevates the combustion temperature of the material and lowers maximum weight loss rates. This leads to an upsurge in the production of residue or char.
Other Notes
The most commonly used salt for the fractional precipitation of proteins due to its solubility and effectiveness; Protein crystallization; Sepharose™ chromatography of tRNAs using reverse salt gradients.
Legal Information
Sepharose is a trademark of Cytiva
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T.D. Pollard
Methods in Enzymology, 85, 334-334 (1982)
Sepharose chromatography of transfer ribonucleic acids using reverse salt gradients.
G W Hatfield
Methods in enzymology, 59, 215-218 (1979-01-01)
Protein crystallization: the growth of large-scale single crystals.
G L Gilliland et al.
Methods in enzymology, 104, 370-381 (1984-01-01)
James F Hunter et al.
Environmental science & technology, 48(17), 10227-10234 (2014-08-06)
A large number of organic species emitted into the atmosphere contain cycloalkyl groups. While cyclic species are believed to be important secondary organic aerosol (SOA) precursors, the specific role of cyclic moieties (particularly for species with multiple or fused rings)
Kazunori Sugiura et al.
Biochemical and biophysical research communications, 457(3), 242-248 (2015-01-17)
Intracellular redox state is a critical factor for fundamental cellular functions, including regulation of the activities of various metabolic enzymes as well as ROS production and elimination. Genetically-encoded fluorescent redox sensors, such as roGFP (Hanson, G. T., et al. (2004))
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service